Clinical Practice Associated with a Switch from and to Ziprasidone during Routine Inpatient Treatment of Patients with Schizophrenia
- PMID: 22937263
- PMCID: PMC3420656
- DOI: 10.1155/2011/317368
Clinical Practice Associated with a Switch from and to Ziprasidone during Routine Inpatient Treatment of Patients with Schizophrenia
Abstract
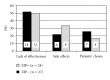
Ziprasidone (ZIP) shows a low propensity for metabolic side effects but can prolong QTc time. It is unclear how these features translate into clinical reality. Charts of inpatients with schizophrenia and switched from (ZIP - , n = 27) or to ZIP (ZIP + , n = 24) were reviewed. Clinical data including documented switch reasons were anonymously analyzed. Comorbidity, body mass index (BMI) at admission, illness severity, side effects, illness duration, and length of stay were comparable in both groups. About 2/3 of ZIP+ were women (1/3 of ZIP - , P = 0.035); ZIP+ patients were younger (P = 0.017), had higher BMI values (P = 0.042), and received higher chlorpromazine equivalents before switch (P = 0.004) whereas ZIP doses were comparable (136 versus 141 mg/d). More patients in ZIP- versus ZIP+ were switched because of previous weight gain (P = 0.006) and depression (P = 0.085) whereas single reasons for ZIP- versus ZIP+ were mainly persisting positive symptoms (P = 0.089) and patients' choice (P = 0.10). The results of the naturalistic study corroborate controlled trials.
Figures
References
-
- Seeger TF, Seymour PA, Schmidt AW, et al. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. Journal of Pharmacology and Experimental Therapeutics. 1995;275(1):101–113. - PubMed
-
- Schotte A, Janssen PFM, Gommeren W, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124(1-2):57–73. - PubMed
-
- Kim SW, Shin IS, Kim JM, Bae KY, Yang SJ, Yoon JS. Effectiveness of switching from aripiprazole to ziprasidone in patients with schizophrenia. Clinical Neuropharmacology. 2010;33(3):121–125. - PubMed
LinkOut - more resources
Full Text Sources