Antibiotics for bronchiectasis exacerbations in children: rationale and study protocol for a randomised placebo-controlled trial
- PMID: 22937736
- PMCID: PMC3488323
- DOI: 10.1186/1745-6215-13-156
Antibiotics for bronchiectasis exacerbations in children: rationale and study protocol for a randomised placebo-controlled trial
Abstract
Background: Despite bronchiectasis being increasingly recognised as an important cause of chronic respiratory morbidity in both indigenous and non-indigenous settings globally, high quality evidence to inform management is scarce. It is assumed that antibiotics are efficacious for all bronchiectasis exacerbations, but not all practitioners agree. Inadequately treated exacerbations may risk lung function deterioration. Our study tests the hypothesis that both oral azithromycin and amoxicillin-clavulanic acid are superior to placebo at improving resolution rates of respiratory exacerbations by day 14 in children with bronchiectasis unrelated to cystic fibrosis.
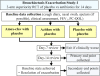
Methods: We are conducting a bronchiectasis exacerbation study (BEST), which is a multicentre, randomised, double-blind, double-dummy, placebo-controlled, parallel group trial, in five centres (Brisbane, Perth, Darwin, Melbourne, Auckland). In the component of BEST presented here, 189 children fulfilling inclusion criteria are randomised (allocation-concealed) to receive amoxicillin-clavulanic acid (22.5 mg/kg twice daily) with placebo-azithromycin; azithromycin (5 mg/kg daily) with placebo-amoxicillin-clavulanic acid; or placebo-azithromycin with placebo-amoxicillin-clavulanic acid for 14 days. Clinical data and a paediatric cough-specific quality of life score are obtained at baseline, at the start and resolution of exacerbations, and at day 14. In most children, blood and deep nasal swabs are also collected at the same time points. The primary outcome is the proportion of children whose exacerbations have resolved at day 14. The main secondary outcome is the paediatric cough-specific quality of life score. Other outcomes are time to next exacerbation; requirement for hospitalisation; duration of exacerbation; and spirometry data. Descriptive viral and bacteriological data from nasal samples and blood markers will also be reported.
Discussion: Effective, evidence-based management of exacerbations in people with bronchiectasis is clinically important. Yet, there are few randomised controlled trials (RCTs) in the neglected area of non-cystic fibrosis bronchiectasis. Indeed, no published RCTs addressing the treatment of bronchiectasis exacerbations in children exist. Our multicentre, double-blind RCT is designed to determine if azithromycin and amoxicillin-clavulanic acid, compared with placebo, improve symptom resolution on day 14 in children with acute respiratory exacerbations. Our planned assessment of the predictors of antibiotic response, the role of antibiotic-resistant respiratory pathogens, and whether early treatment with antibiotics affects duration and time to the next exacerbation, are also all novel.
Trial registration: Australia and New Zealand Clinical Trials Register (ANZCTR) number ACTRN12612000011886.
Figures
References
-
- Chang AB, Grimwood K, Macguire G, King PT, Morris PS, Torzillo PJ. Management of bronchiectasis and chronic suppurative lung disease (CSLD) in Indigenous children and adults from rural and remote Australian communities. Med J Aust. 2008;189:386–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical