Contribution of GATA1 dysfunction to multi-step leukemogenesis
- PMID: 22937757
- PMCID: PMC7659349
- DOI: 10.1111/cas.12007
Contribution of GATA1 dysfunction to multi-step leukemogenesis
Abstract
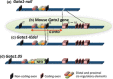
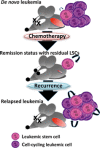
In mammals, hematopoietic homeostasis is maintained by a fine-tuned balance among the self-renewal, proliferation, differentiation and survival of hematopoietic stem cells and their progenies. Each process is also supported by the delicate balance of the expression of multiple genes specific to each process. GATA1 is a transcription factor that comprehensively regulates the genes that are important for the development of erythroid and megakaryocytic cells. Accumulating evidence supports the notion that defects in GATA1 function are intimately linked to hematopoietic disorders. In particular, the somatic mutation of the GATA1 gene, which leads to the production of N-terminally truncated GATA1, contributes to the genesis of transient myeloproliferative disorder and acute megakaryoblastic leukemia in infants with Down syndrome. Similarly, a mutation in the GATA1 regulatory region that reduces GATA1 expression is involved in the onset of erythroid leukemia in mice. In both cases, the accumulation of immature progenitor cells caused by GATA1 dysregulation underlies the pathogenesis of the leukemia. This review provides a summary of multi-step leukemogenesis with a focus on GATA1 dysfunction.
© 2012 Japanese Cancer Association.
Figures


References
-
- Tsai SF, Martin DI, Zon LI, D'Andrea AD, Wong GG, Orkin SH. Cloning of cDNA for the major DNA‐binding protein of the erythroid lineage through expression in mammalian cells. Nature 1989; 339: 446–51. - PubMed
-
- Yamamoto M, Ko LJ, Leonard MW, Beug H, Orkin SH, Engel JD. Activity and tissue‐specific expression of the transcription factor NF‐E1 multigene family. Genes Dev 1990; 4: 1650–62. - PubMed
-
- Shimizu R, Yamamoto M. Gene expression regulation and domain function of hematopoietic GATA factors. Semin Cell Dev Biol 2005; 16: 129–36. - PubMed
-
- Zheng W, Flavell RA. The transcription factor GATA‐3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997; 89: 587–96. - PubMed
-
- Suzuki M, Shimizu R, Yamamoto M. Transcriptional regulation by GATA1 and GATA2 during erythropoiesis. Int J Hematol 2011; 93: 150–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

