Redox and reactive oxygen species regulation of mitochondrial cytochrome C oxidase biogenesis
- PMID: 22937827
- PMCID: PMC3852343
- DOI: 10.1089/ars.2012.4847
Redox and reactive oxygen species regulation of mitochondrial cytochrome C oxidase biogenesis
Abstract
Significance: Cytochrome c oxidase (COX), the last enzyme of the mitochondrial respiratory chain, is the major oxygen consumer enzyme in the cell. COX biogenesis involves several redox-regulated steps. The process is highly regulated to prevent the formation of pro-oxidant intermediates.
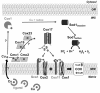
Recent advances: Regulation of COX assembly involves several reactive oxygen species and redox-regulated steps. These include: (i) Intricate redox-controlled machineries coordinate the expression of COX isoenzymes depending on the environmental oxygen concentration. (ii) COX is a heme A-copper metalloenzyme. COX copper metallation involves the copper chaperone Cox17 and several other recently described cysteine-rich proteins, which are oxidatively folded in the mitochondrial intermembrane space. Copper transfer to COX subunits 1 and 2 requires concomitant transfer of redox power. (iii) To avoid the accumulation of reactive assembly intermediates, COX is regulated at the translational level to minimize synthesis of the heme A-containing Cox1 subunit when assembly is impaired.
Critical issues: An increasing number of regulatory pathways converge to facilitate efficient COX assembly, thus preventing oxidative stress.
Future directions: Here we will review on the redox-regulated COX biogenesis steps and will discuss their physiological relevance. Forthcoming insights into the precise regulation of mitochondrial COX biogenesis in normal and stress conditions will likely open future perspectives for understanding mitochondrial redox regulation and prevention of oxidative stress.
Figures






References
-
- Acin-Perez R. Fernandez-Silva P. Peleato ML. Perez-Martos A. Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32:529–539. - PubMed
-
- Allen LA. Zhao XJ. Caughey W. Poyton RO. Isoforms of yeast cytochrome c oxidase subunit V affect the binuclear reaction center and alter the kinetics of interaction with the isoforms of yeast cytochrome c. J Biol Chem. 1995;270:110–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

