Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells
- PMID: 22937862
- PMCID: PMC3442961
- DOI: 10.1186/1746-6148-8-150
Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells
Abstract
Background: Bone marrow-derived mesenchymal stem cells (BM-MSCs) and adipose tissue-derived mesenchymal stem cells (AT-MSCs) are potential cellular sources of therapeutic stem cells. MSCs are a multipotent population of cells capable of differentiating into a number of mesodermal lineages. Treatment using MSCs appears to be a helpful approach for structural restoration in regenerative medicine. Correct identification of these cells is necessary, but there is inadequate information on the MSC profile of cell surface markers and mRNA expression in dogs. In this study, we performed molecular characterization of canine BM-MSCs and AT-MSCs using immunological and mRNA expression analysis.
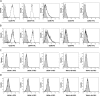
Results: Samples were confirmed to be multipotent based on their osteogenic and adipogenic differentiation. And these cells were checked as stem cell, hematopoietic and embryonic stem cell (ESC) markers by flow cytometry. BM- and AT-MSCs showed high expression of CD29 and CD44, moderate expression of CD90, and were negative for CD34, CD45, SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81. SSEA-1 was expressed at very low levels in AT-MSCs. Quantitative real-time PCR (qRT-PCR) revealed expression of Oct3/4, Sox2, and Nanog in BM- and AT-MSCs. There was no significant difference in expression of Oct3/4 and Sox2 between BM-MSCs and AT-MSCs. However, Nanog expression was 2.5-fold higher in AT-MSCs than in BM-MSCs. Using immunocytochemical analysis, Oct3/4 and Sox2 proteins were observed in BM- and AT-MSCs.
Conclusion: Our results provide fundamental information to enable for more reproducible and reliable quality control in the identification of canine BM-MSCs and AT-MSCs by protein and mRNA expression analysis.
Figures

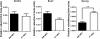

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

