Identification of the Streptococcus mutans LytST two-component regulon reveals its contribution to oxidative stress tolerance
- PMID: 22937869
- PMCID: PMC3507848
- DOI: 10.1186/1471-2180-12-187
Identification of the Streptococcus mutans LytST two-component regulon reveals its contribution to oxidative stress tolerance
Abstract
Background: The S. mutans LrgA/B holin-like proteins have been shown to affect biofilm formation and oxidative stress tolerance, and are regulated by oxygenation, glucose levels, and by the LytST two-component system. In this study, we sought to determine if LytST was involved in regulating lrgAB expression in response to glucose and oxygenation in S. mutans.
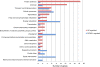
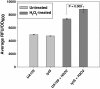
Results: Real-time PCR revealed that growth phase-dependent regulation of lrgAB expression in response to glucose metabolism is mediated by LytST under low-oxygen conditions. However, the effect of LytST on lrgAB expression was less pronounced when cells were grown with aeration. RNA expression profiles in the wild-type and lytS mutant strains were compared using microarrays in early exponential and late exponential phase cells. The expression of 40 and 136 genes in early-exponential and late exponential phase, respectively, was altered in the lytS mutant. Although expression of comYB, encoding a DNA binding-uptake protein, was substantially increased in the lytS mutant, this did not translate to an effect on competence. However, a lrgA mutant displayed a substantial decrease in transformation efficiency, suggestive of a previously-unknown link between LrgA and S. mutans competence development. Finally, increased expression of genes encoding antioxidant and DNA recombination/repair enzymes was observed in the lytS mutant, suggesting that the mutant may be subjected to increased oxidative stress during normal growth. Although the intracellular levels of reaction oxygen species (ROS) appeared similar between wild-type and lytS mutant strains after overnight growth, challenge of these strains with hydrogen peroxide (H2O2) resulted in increased intracellular ROS in the lytS mutant.
Conclusions: Overall, these results: (1) Reinforce the importance of LytST in governing lrgAB expression in response to glucose and oxygen, (2) Define a new role for LytST in global gene regulation and resistance to H2O2, and (3) Uncover a potential link between LrgAB and competence development in S. mutans.
Figures



References
-
- Biswas S, Bowler IC, Bunch C, Prendergast B, Webster DP. Streptococcus mutans infective endocarditis complicated by vertebral discitis following dental treatment without antibiotic prophylaxis. J Med Microbiol. 2010;59(Pt 10):1257–1259. - PubMed
-
- Ullman RF, Miller SJ, Strampfer MJ, Cunha BA. Streptococcus mutans endocarditis: report of three cases and review of the literature. Heart Lung. 1988;17(2):209–212. - PubMed
-
- Vose JM, Smith PW, Henry M, Colan D. Recurrent Streptococcus mutans endocarditis. Am J Med. 1987;82(3 Spec No):630–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

