Mitochondrial plasticity in obesity and diabetes mellitus
- PMID: 22938510
- PMCID: PMC3691915
- DOI: 10.1089/ars.2012.4910
Mitochondrial plasticity in obesity and diabetes mellitus
Abstract
Significance: Insulin resistance and its related diseases, obesity and type 2 diabetes mellitus (T2DM), have been linked to changes in aerobic metabolism, pointing to a possible role of mitochondria in the development of insulin resistance.
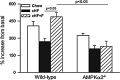
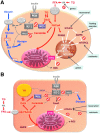
Recent advances: Refined methodology of ex vivo high-resolution respirometry and in vivo magnetic resonance spectroscopy now allows describing several features of mitochondria in humans. In addition to measuring mitochondrial function at baseline and after exercise-induced submaximal energy depletion, the response of mitochondria to endocrine and metabolic challenges, termed mitochondrial plasticity, can be assessed using hyperinsulinemic clamp tests. While insulin resistant states do not uniformly relate to baseline and post-exercise mitochondrial function, mitochondrial plasticity is typically impaired in insulin resistant relatives of T2DM, in overt T2DM and even in type 1 diabetes mellitus (T1DM).
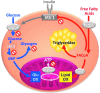
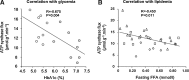
Critical issues: The variability of baseline mitochondrial function in the main target tissue of insulin action, skeletal muscle and liver, may be attributed to inherited and acquired changes in either mitochondrial quantity or quality. In addition to certain gene polymorphisms and aging, circulating glucose and lipid concentrations correlate with both mitochondrial function and plasticity.
Future directions: Despite the associations between features of mitochondrial function and insulin sensitivity, the question of a causal relationship between compromised mitochondrial plasticity and insulin resistance in the development of obesity and T2DM remains to be resolved.
Figures




Similar articles
-
Alterations of Mitochondrial Function and Insulin Sensitivity in Human Obesity and Diabetes Mellitus.Annu Rev Nutr. 2016 Jul 17;36:337-67. doi: 10.1146/annurev-nutr-071715-050656. Epub 2016 May 4. Annu Rev Nutr. 2016. PMID: 27146012 Review.
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
The role of weight loss and exercise in correcting skeletal muscle mitochondrial abnormalities in obesity, diabetes and aging.Mol Cell Endocrinol. 2013 Oct 15;379(1-2):30-4. doi: 10.1016/j.mce.2013.06.018. Epub 2013 Jun 20. Mol Cell Endocrinol. 2013. PMID: 23792186 Review.
-
The role of mitochondria in insulin resistance and type 2 diabetes mellitus.Nat Rev Endocrinol. 2011 Sep 13;8(2):92-103. doi: 10.1038/nrendo.2011.138. Nat Rev Endocrinol. 2011. PMID: 21912398 Review.
-
Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects.Diabetologia. 2007 Jan;50(1):113-20. doi: 10.1007/s00125-006-0475-1. Epub 2006 Nov 9. Diabetologia. 2007. PMID: 17093944
Cited by
-
Adenosine Triphosphate Production of Muscle Mitochondria after Acute Exercise in Lean and Obese Humans.Med Sci Sports Exerc. 2019 Mar;51(3):445-453. doi: 10.1249/MSS.0000000000001812. Med Sci Sports Exerc. 2019. PMID: 30363008 Free PMC article.
-
LC-MS-Based Lipidomic Analysis of Serum Samples from Patients with Type 2 Diabetes Mellitus (T2DM).Dis Markers. 2022 Feb 12;2022:5559470. doi: 10.1155/2022/5559470. eCollection 2022. Dis Markers. 2022. PMID: 35190756 Free PMC article.
-
Mitochondria and cardiovascular diseases-from pathophysiology to treatment.Ann Transl Med. 2018 Jun;6(12):256. doi: 10.21037/atm.2018.06.21. Ann Transl Med. 2018. PMID: 30069458 Free PMC article. Review.
-
Sex-Specific Skeletal Muscle Fatigability and Decreased Mitochondrial Oxidative Capacity in Adult Rats Exposed to Postnatal Hyperoxia.Front Physiol. 2018 Mar 29;9:326. doi: 10.3389/fphys.2018.00326. eCollection 2018. Front Physiol. 2018. PMID: 29651255 Free PMC article.
-
Effect of sericin on diabetic hippocampal growth hormone/insulin-like growth factor 1 axis.Neural Regen Res. 2013 Jul 5;8(19):1756-64. doi: 10.3969/j.issn.1673-5374.2013.19.003. Neural Regen Res. 2013. PMID: 25206472 Free PMC article.
References
-
- Abdul-Ghani MA. Muller FL. Liu Y. Chavez AO. Balas B. Zuo P. Chang Z. Tripathy D. Jani R. Molina-Carrion M. Monroy A. Folli F. Van Remmen H. DeFronzo RA. Deleterious action of FA metabolites on ATP synthesis: Possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E678–685. - PubMed
-
- Bass A. Vondra K. Rath R. Vitek V. Havranek T. Metabolic changes in the quadriceps femoris muscle of obese people. Enzyme activity patterns of energy-supplying metabolism. Pflugers Arch. 1975;359:325–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

