Effect of inhaled glucocorticoids in childhood on adult height
- PMID: 22938716
- PMCID: PMC3517799
- DOI: 10.1056/NEJMoa1203229
Effect of inhaled glucocorticoids in childhood on adult height
Abstract
Background: The use of inhaled glucocorticoids for persistent asthma causes a temporary reduction in growth velocity in prepubertal children. The resulting decrease in attained height 1 to 4 years after the initiation of inhaled glucocorticoids is thought not to decrease attained adult height.
Methods: We measured adult height in 943 of 1041 participants (90.6%) in the Childhood Asthma Management Program; adult height was determined at a mean (±SD) age of 24.9±2.7 years. Starting at the age of 5 to 13 years, the participants had been randomly assigned to receive 400 μg of budesonide, 16 mg of nedocromil, or placebo daily for 4 to 6 years. We calculated differences in adult height for each active treatment group, as compared with placebo, using multiple linear regression with adjustment for demographic characteristics, asthma features, and height at trial entry.
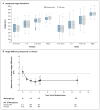
Results: Mean adult height was 1.2 cm lower (95% confidence interval [CI], -1.9 to -0.5) in the budesonide group than in the placebo group (P=0.001) and was 0.2 cm lower (95% CI, -0.9 to 0.5) in the nedocromil group than in the placebo group (P=0.61). A larger daily dose of inhaled glucocorticoid in the first 2 years was associated with a lower adult height (-0.1 cm for each microgram per kilogram of body weight) (P=0.007). The reduction in adult height in the budesonide group as compared with the placebo group was similar to that seen after 2 years of treatment (-1.3 cm; 95% CI, -1.7 to -0.9). During the first 2 years, decreased growth velocity in the budesonide group occurred primarily in prepubertal participants.
Conclusions: The initial decrease in attained height associated with the use of inhaled glucocorticoids in prepubertal children persisted as a reduction in adult height, although the decrease was not progressive or cumulative. (Funded by the National Heart, Lung, and Blood Institute and the National Center for Research Resources; CAMP ClinicalTrials.gov number, NCT00000575.).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Inhaled glucocorticoids and adult height.N Engl J Med. 2012 Nov 29;367(22):2156; author reply 2156-7. doi: 10.1056/NEJMc1211948. N Engl J Med. 2012. PMID: 23190233 No abstract available.
-
It is still an open question whether inhaled glucocorticoid-induced effects may reduce adult height.Evid Based Med. 2013 Oct;18(5):195-6. doi: 10.1136/eb-2012-101074. Epub 2013 Jan 19. Evid Based Med. 2013. PMID: 23335270 No abstract available.
-
Inhaled steroid controller-medication use in childhood has a negative effect on final adult height.J Pediatr. 2013 Mar;162(3):650-1. doi: 10.1016/j.jpeds.2012.12.060. J Pediatr. 2013. PMID: 23438921 No abstract available.
-
This asthma treatment has a lasting side effect in children.J Fam Pract. 2013 Sep;62(9):500-2. J Fam Pract. 2013. PMID: 24080559 Free PMC article.
References
-
- Expert Panel Report 3 (EPR3): guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. ( http://www.nhlbi.nih.gov/guidelines/asthma.
-
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–63. - PubMed
-
- Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomized, double-blind trial. Lancet. 2003;361:1071–6. - PubMed
-
- Sharek PJ, Bergman DA. The effect of inhaled steroids on linear growth of children with asthma: a meta-analysis. Pediatrics. 2000;106(1):e8. - PubMed
-
- Ferguson AC, Van Bever HP, Teper AM, Lasytsya O, Goldfrad CH, Whitehead PJ. A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respir Med. 2007;101:118–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01-HR-16046/HR/NHLBI NIH HHS/United States
- N01-HR-16048/HR/NHLBI NIH HHS/United States
- N01 HR016044/HL/NHLBI NIH HHS/United States
- U01HL075420/HL/NHLBI NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- N01-HR-16045/HR/NHLBI NIH HHS/United States
- N01-HR-16052/HR/NHLBI NIH HHS/United States
- U01 HL075416/HL/NHLBI NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- U01 HL075408/HL/NHLBI NIH HHS/United States
- U01 HL075232/HL/NHLBI NIH HHS/United States
- U01 HL075415/HL/NHLBI NIH HHS/United States
- U01 HL075409/HL/NHLBI NIH HHS/United States
- U01HL075232/HL/NHLBI NIH HHS/United States
- U01HL075409/HL/NHLBI NIH HHS/United States
- N01-HR-16049/HL/NHLBI NIH HHS/United States
- RR00036/RR/NCRR NIH HHS/United States
- U01HL075407/HL/NHLBI NIH HHS/United States
- N01-HR-16051/HR/NHLBI NIH HHS/United States
- U01 HL075407/HL/NHLBI NIH HHS/United States
- M01 RR002719/RR/NCRR NIH HHS/United States
- U01 HL075420/HL/NHLBI NIH HHS/United States
- U01HL075416/HL/NHLBI NIH HHS/United States
- N01-HR-16044/HR/NHLBI NIH HHS/United States
- U01HL075415/HL/NHLBI NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- M01RR02719-14/RR/NCRR NIH HHS/United States
- U01 HL075417/HL/NHLBI NIH HHS/United States
- U01 HL075419/HL/NHLBI NIH HHS/United States
- U01HL075419/HL/NHLBI NIH HHS/United States
- N01-HR-16050/HR/NHLBI NIH HHS/United States
- U01HL075408/HL/NHLBI NIH HHS/United States
- N01-HR-16047/HR/NHLBI NIH HHS/United States
- U01HL075417/HL/NHLBI NIH HHS/United States
- M01RR00051/RR/NCRR NIH HHS/United States
- M01RR0099718-24/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
