Abnormalities of calcium handling proteins in skeletal muscle mirror those of the heart in humans with heart failure: a shared mechanism?
- PMID: 22939042
- PMCID: PMC3437990
- DOI: 10.1016/j.cardfail.2012.07.005
Abnormalities of calcium handling proteins in skeletal muscle mirror those of the heart in humans with heart failure: a shared mechanism?
Abstract
Background: In the failing human heart, abnormalities of Ca(2+) cycling have been described, but there is scant knowledge about Ca(2+) handling in the skeletal muscle of humans with heart failure (HF). We tested the hypothesis that in humans with HF, Ca(2+) cycling proteins in skeletal muscle are abnormal.
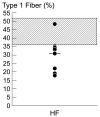
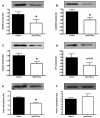
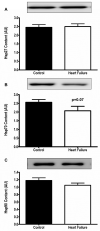
Methods and results: Ten advanced HF patients (50.4 ± 3.7 years), and 9 age-matched controls underwent vastus lateralis biopsy. Western blot analysis showed that sarco(endo)plasmic reticulum Ca(2+)-ATPase (SERCA)2a, which is responsible for Ca(2+) sequestration into the sarcoplasmic reticulum(SR), was lower in HF versus controls (4.8 ± 0.5 vs 7.5 ± 0.8 AU, P = .01). Although phospholamban (PLN), which inhibits SERCA2a, was not different in HF versus controls, phosphorylation (SER16 site) of PLN, which relieves this inhibition, was reduced (0.8 ± 0.1 vs 3.9 ± 0.9 AU, P = .004). Dihydropyridine receptors were reduced in HF, (2.1 ± 0.4 vs 3.6 ± 0.5 AU, P = .04). We tested the hypothesis that these abnormalities of Ca(2+) handling protein content and regulation were due to increased oxidative stress, but oxygen radical scavenger proteins were not elevated in the skeletal muscle of HF patients.
Conclusion: In chronic HF, marked abnormalities of Ca(2+) handling proteins are present in skeletal muscle, which mirror those in failing heart tissue. This suggests a common mechanism, such as chronic augmentation of sympathetic activity and autophosphorylation of Ca(2+)-calmodulin-dependent-protein kinase II.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indices of resting left ventricular performance in heart failure. Am J Cardiol. 1991;47:33–9. - PubMed
-
- Higginbotham M, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51:51–2. - PubMed
-
- Maskin CS, Forman R, Sonnenblick EH, Frishman WH, LeJemtel TH. Failure of dobutamine to increase exercise capacity despite hemodynamic improvement in severe chronic heart failure. Am J Cardiol. 1983;51:177–82. - PubMed
-
- Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, et al. Skeletal muscle function and its relation to exercise tolerance in chronic HF. J Am Coll Cardiol. 1997;30:1758–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

