West Nile virus growth is independent of autophagy activation
- PMID: 22939285
- PMCID: PMC3444629
- DOI: 10.1016/j.virol.2012.08.016
West Nile virus growth is independent of autophagy activation
Abstract
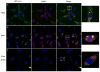
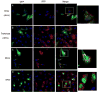
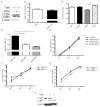
West Nile virus (WNV) is an arthropod-borne virus with a worldwide distribution that causes neurologic disease and death. Autophagy is a cellular homeostatic mechanism involved in antiviral responses but can be subverted to support viral growth as well. We show that autophagy is induced by WNV infection in cell culture and in primary neuron cultures. Following WNV infection, lysosomes co-localize with autophagosomes resulting in LC3B-II turnover and autolysosomal acidification. However, activation or inhibition of autophagy has no significant effect on WNV growth but pharmacologic inhibition of PI3 kinases associated with autophagy reduce WNV growth. Basal levels of p62/sequestosome1(SQSTM1) do not significantly change following WNV-induced autophagy activation, but p62 is turned over or degraded by autophagy activation implying that p62 expression is increased following WNV-infection. These data show that WNV-induces autophagy but viral growth is independent of autophagy activation suggesting that WNV-specific interactions with autophagy have diverged from other flaviviruses.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

