Cellular uptake and processing of enamel matrix derivative by human periodontal ligament fibroblasts
- PMID: 22939369
- PMCID: PMC3587288
- DOI: 10.1016/j.archoralbio.2012.08.003
Cellular uptake and processing of enamel matrix derivative by human periodontal ligament fibroblasts
Abstract
Objective: Enamel matrix derivative (EMD), is an extract of porcine developing enamel matrix. Its commercialised form Emdogain, is claimed to stimulate periodontal regeneration by recapitulating original developmental processes, although the mechanism remains unclear. Our objective was to investigate interactions between EMD and human periodontal ligament (HPDL) fibroblasts in vitro.
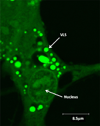
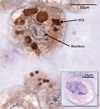
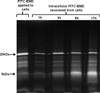
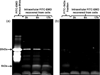
Design: HPDL fibroblasts were cultured in the presence of fluorescently labelled EMD and cellular EMD uptake was monitored using confocal laser scanning microscopy and immunohistochemistry. Internalised EMD proteins were characterised using SDS-PAGE.
Results: EMD was internalised by HPDL fibroblasts leading to the appearance of multiple, vesicle-like structure in the cytoplasm. The internalised protein was composed mainly of the major 20kDa amelogenin component of EMD which was subsequently processed with time to generate a cumulative 5kDa component.
Conclusions: Cellular uptake and subsequent intracellular processing of EMD components by dental mesenchymal cells may play a role in EMD bioactivity and in part explain the turnover of Emdogain when placed clinically.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Gingival crevicular fluid can degrade Emdogain and inhibit Emdogain-induced proliferation of periodontal ligament fibroblasts.J Periodontal Res. 2010 Jun;45(3):353-60. doi: 10.1111/j.1600-0765.2009.01244.x. Epub 2009 Nov 9. J Periodontal Res. 2010. PMID: 19909398
-
Human periodontal fibroblast response to enamel matrix derivative, amelogenin, and platelet-derived growth factor-BB.J Periodontol. 2006 Jul;77(7):1242-52. doi: 10.1902/jop.2006.050147. J Periodontol. 2006. PMID: 16805689
-
Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative.J Clin Periodontol. 2001 Feb;28(2):181-8. doi: 10.1034/j.1600-051x.2001.028002181.x. J Clin Periodontol. 2001. PMID: 11168744
-
Enamel matrix derivative: a review of cellular effects in vitro and a model of molecular arrangement and functioning.Tissue Eng Part B Rev. 2012 Jun;18(3):181-202. doi: 10.1089/ten.TEB.2011.0365. Epub 2011 Dec 28. Tissue Eng Part B Rev. 2012. PMID: 22070552 Review.
-
Twenty years of enamel matrix derivative: the past, the present and the future.J Clin Periodontol. 2016 Aug;43(8):668-83. doi: 10.1111/jcpe.12546. Epub 2016 May 28. J Clin Periodontol. 2016. PMID: 26987551 Review.
Cited by
-
Single-cell sequencing systematically analyzed the mechanism of Emdogain on the restoration of delayed replantation periodontal membrane.Int J Oral Sci. 2025 Apr 17;17(1):33. doi: 10.1038/s41368-024-00345-5. Int J Oral Sci. 2025. PMID: 40246878 Free PMC article.
-
Emdogain-regulated gene expression in palatal fibroblasts requires TGF-βRI kinase signaling.PLoS One. 2014 Sep 8;9(9):e105672. doi: 10.1371/journal.pone.0105672. eCollection 2014. PLoS One. 2014. PMID: 25197981 Free PMC article.
References
-
- Straumann The regenerative approach. Available from: http://www.straumann.co.uk/gb-index/products/products-biologics/products... [cited 13.04.12]
-
- Gestrelius S, Andersson C, Lidstrom D, Hammarstrom L, Somerman M. In vitro studies on periodontal ligament cells and enamel matrix derivative. Journal of Clinical Periodontology. 1997;24(9 Pt 2):685–692. - PubMed
-
- Maycock J, Wood SR, Brookes SJ, Shore RC, Robinson C, Kirkham J. Characterization of a porcine amelogenin preparation EMDOGAIN, a biological treatment for periodontal disease. Connective Tissue Research. 2002;43(2–3):472–476. - PubMed
-
- Viswanathan HL, Berry JE, Foster BL, Gibson CW, Li Y, Kulkarni AB, et al. Amelogenin: a potential regulator of cementum-associated genes. Journal of Periodontology. 2003;74(10):1423–1431. - PubMed
-
- Tompkins K, Alvares K, George A, Veis A. Two related low molecular mass polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ differentiation in vitro. Journal of Bone and Mineral Research. 2005;20(2):341–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

