Lis1 acts as a "clutch" between the ATPase and microtubule-binding domains of the dynein motor
- PMID: 22939623
- PMCID: PMC3438448
- DOI: 10.1016/j.cell.2012.07.022
Lis1 acts as a "clutch" between the ATPase and microtubule-binding domains of the dynein motor
Abstract
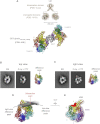
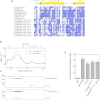
The lissencephaly protein Lis1 has been reported to regulate the mechanical behavior of cytoplasmic dynein, the primary minus-end-directed microtubule motor. However, the regulatory mechanism remains poorly understood. Here, we address this issue using purified proteins from Saccharomyces cerevisiae and a combination of techniques, including single-molecule imaging and single-particle electron microscopy. We show that rather than binding to the main ATPase site within dynein's AAA+ ring or its microtubule-binding stalk directly, Lis1 engages the interface between these elements. Lis1 causes individual dynein motors to remain attached to microtubules for extended periods, even during cycles of ATP hydrolysis that would canonically induce detachment. Thus, Lis1 operates like a "clutch" that prevents dynein's ATPase domain from transmitting a detachment signal to its track-binding domain. We discuss how these findings provide a conserved mechanism for dynein functions in living cells that require prolonged microtubule attachments.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
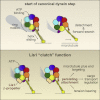
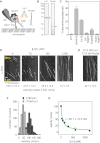

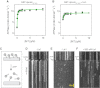
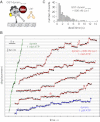
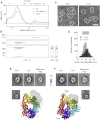

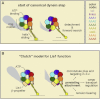
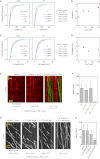

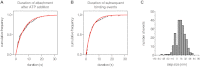
Comment in
-
LIS1 clamps dynein to the microtubule.Cell. 2012 Aug 31;150(5):877-9. doi: 10.1016/j.cell.2012.08.010. Cell. 2012. PMID: 22939616
References
-
- Allan V.J. Cytoplasmic dynein. Biochem. Soc. Trans. 2011;39:1169–1178. - PubMed
-
- Burgess S.A., Walker M.L., Sakakibara H., Knight P.J., Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. - PubMed
-
- Delanoue R., Davis I. Dynein anchors its mRNA cargo after apical transport in the Drosophila blastoderm embryo. Cell. 2005;122:97–106. - PubMed
Supplemental References
-
- Case, R.B., Pierce, D.W., Hom-Booher, N., Hart, C.L., and Vale, R.D. (1997). The directional preference of kinesin motors is specified by an element outside of the motor catalytic domain. Cell 90, 959–966. - PubMed
-
- Frank, J., Radermacher, M., Penczek, P., Zhu, J., Li, Y., Ladjadj, M., and Leith, A. (1996). SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199. - PubMed
-
- Guthrie, C., and Fink, G.R., eds. (1991). Guide to yeast genetics and molecular biology (San Diego: Academic Press).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

