Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion
- PMID: 22940097
- PMCID: PMC3456987
- DOI: 10.1016/j.immuni.2012.05.029
Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion
Abstract
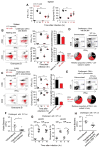
Memory CD8(+) T cells induced upon immunization exhibit improved functional features that contribute to protection of immunized hosts. Although both cognate antigen recognition and inflammation are important for memory CD8(+) T cell reactivation, the relative contribution of these factors and the cell types providing these signals in vivo are poorly defined. Here, we show that Ly6C(+)CCR2(+) inflammatory monocytes, a subset of monocytes, largely orchestrate memory CD8(+) T and NK lymphocytes activation by differentiating into interleukin-18 (IL-18)- and IL-15-producing cells in an inflammasome and type I interferon-IRF3-dependent manner. Memory CD8(+) T cells became potent effector cells by sensing inflammation from monocytes independently of their cognate antigen. Like NK cells, they underwent rapid mobilization, upregulated intense and sustained effector functions during bacterial, viral, and parasitic infections, and contributed to innate responses and protection in vivo. Thus, inflammatory monocyte-derived IL-18 and IL-15 are critical to initiate memory CD8(+) T and NK lymphocytes differentiation into antimicrobial effector cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

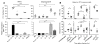




Comment in
-
T cell responses: Dancing in the dark.Nat Rev Immunol. 2012 Oct;12(10):684-5. doi: 10.1038/nri3310. Epub 2012 Sep 14. Nat Rev Immunol. 2012. PMID: 22976436 No abstract available.
-
Indiscriminate memories during infection control.Immunity. 2012 Sep 21;37(3):445-6. doi: 10.1016/j.immuni.2012.09.003. Immunity. 2012. PMID: 22999951
References
-
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. - PubMed
-
- Beuneu H, Deguine J, Bouvier I, Di Santo JP, Albert ML, Bousso P. A dual role for type I IFNs during polyinosinic-polycytidylic acid-induced NK cell activation. J Immunol. 2011;187:2084–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

