HIV immune complexes prevent excitotoxicity by interaction with NMDA receptors
- PMID: 22940423
- PMCID: PMC3556339
- DOI: 10.1016/j.nbd.2012.08.013
HIV immune complexes prevent excitotoxicity by interaction with NMDA receptors
Abstract
Purpose: Human immunodeficiency virus-1 (HIV)-associated neurocognitive disorder (HAND) is a neurodegenerative disease for which there is no available neuroprotective therapy. Viral proteins, such as Tat, have been implicated as agents of neurotoxicity via multiple mechanisms, including effects by directly binding to the NMDA receptor. We evaluated the ability of the immune response against Tat to modulate neurotoxicity at glutamate receptors.
Methods: Neurotoxicity was measured in primary neuronal-glial cultures and in hippocampal slice cultures. We used immunoprecipitation experiments to demonstrate interaction between Tat, NMDA receptor, and anti-Tat antibody. Using known structures of Tat and NMDA receptors, we developed a model of their interactions.
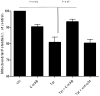
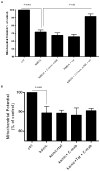
Results: Antibodies to Tat attenuated Tat-mediated neurotoxicity. Interestingly, Tat immune complexes also blocked neurotoxicity caused by NMDA receptor agonists but not kainate/AMPA receptor agonists. Neither Tat nor antibody alone blocked the excitotoxic effect, nor did an unrelated antigen-antibody complex. The protective effect of the Tat immune complexes was also lost when Tat was modified by nitrosylation or by using a deletion mutant of Tat.
Conclusions: The ability of viral immune complexes to interact with NMDA receptors and prevent excitotoxicity represents a novel host defense mechanism. Host immune responses may influence host susceptibility to various effects of viral proteins, modulating HIV complications, such as onset of HAND. These observations provide rationale for development of vaccine therapies targeting Tat for prevention of HAND.
Keywords: Dementia; Glutamate; Neuroprotection; Neurotoxicity; Neurovirology; Neutralizing antibodies.
Published by Elsevier Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures






Similar articles
-
NMDA receptor activation by HIV-Tat protein is clade dependent.J Neurosci. 2008 Nov 19;28(47):12190-8. doi: 10.1523/JNEUROSCI.3019-08.2008. J Neurosci. 2008. PMID: 19020013 Free PMC article.
-
Overactivation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate and N-methyl-D-aspartate but not kainate receptors inhibits phosphatidylcholine synthesis before excitotoxic neuronal death.J Neurochem. 2001 Apr;77(1):13-22. doi: 10.1046/j.1471-4159.2001.t01-2-00187.x. J Neurochem. 2001. PMID: 11279257
-
The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site.Brain Res. 2004 Jan 2;995(1):39-45. doi: 10.1016/j.brainres.2003.09.052. Brain Res. 2004. PMID: 14644469
-
HIV-Associated Neurotoxicity: The Interplay of Host and Viral Proteins.Mediators Inflamm. 2021 Aug 25;2021:1267041. doi: 10.1155/2021/1267041. eCollection 2021. Mediators Inflamm. 2021. PMID: 34483726 Free PMC article. Review.
-
Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders.J Neuroimmune Pharmacol. 2013 Jun;8(3):594-607. doi: 10.1007/s11481-013-9442-z. Epub 2013 Apr 4. J Neuroimmune Pharmacol. 2013. PMID: 23553365 Free PMC article. Review.
Cited by
-
Acute Administration of HIV-1 Tat Protein Drives Glutamatergic Alterations in a Rodent Model of HIV-Associated Neurocognitive Disorders.Mol Neurobiol. 2024 Oct;61(10):8467-8480. doi: 10.1007/s12035-024-04113-8. Epub 2024 Mar 22. Mol Neurobiol. 2024. PMID: 38514527 Free PMC article.
-
Levetiracetam Prevents Neurophysiological Changes and Preserves Cognitive Function in the Human Immunodeficiency Virus (HIV)-1 Transactivator of Transcription Transgenic Mouse Model of HIV-Associated Neurocognitive Disorder.J Pharmacol Exp Ther. 2024 Sep 18;391(1):104-118. doi: 10.1124/jpet.124.002272. J Pharmacol Exp Ther. 2024. PMID: 39060163
-
The Glutamate System as a Crucial Regulator of CNS Toxicity and Survival of HIV Reservoirs.Front Cell Infect Microbiol. 2020 Jun 24;10:261. doi: 10.3389/fcimb.2020.00261. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32670889 Free PMC article. Review.
-
Oligodendrocytes Are Targets of HIV-1 Tat: NMDA and AMPA Receptor-Mediated Effects on Survival and Development.J Neurosci. 2015 Aug 12;35(32):11384-98. doi: 10.1523/JNEUROSCI.4740-14.2015. J Neurosci. 2015. PMID: 26269645 Free PMC article.
-
Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders.J Neurovirol. 2013 Feb;19(1):82-8. doi: 10.1007/s13365-012-0144-8. Epub 2013 Jan 18. J Neurovirol. 2013. PMID: 23329164 Free PMC article.
References
-
- Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain. 2003;126:1058–67. - PubMed
-
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–36. - PubMed
-
- Caporello E, Nath A, Slevin J, Galey D, Hamilton G, Williams L, et al. The immunophilin ligand GPI1046 protects neurons from the lethal effects of the HIV-1 proteins gp120 and Tat by modulating endoplasmic reticulum calcium load. J Neurochem. 2006;98:146–55. - PubMed
-
- Cawley LP, Minard BJ, Tourtellotte WW, Ma BI, Chelle C. Immunofixation electrophoretic techniques applied to identification of proteins in serum and cerebrospinal fluid. Clin Chem. 1976;22:1262–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

