Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice
- PMID: 22940620
- PMCID: PMC3729022
- DOI: 10.1016/j.freeradbiomed.2012.08.009
Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice
Abstract
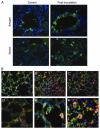
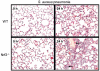
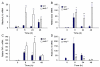
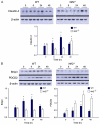
Acute lung injury (ALI) initiates protective responses involving genes downstream of the Nrf2 (Nfe2l2) transcription factor, including heme oxygenase-1 (HO-1), which stimulates mitochondrial biogenesis and related anti-inflammatory processes. We examined mitochondrial biogenesis during Staphylococcus aureus pneumonia in mice and the effect of Nrf2 deficiency on lung mitochondrial biogenesis and resolution of lung inflammation. S. aureus pneumonia established by nasal insufflation of live bacteria was studied in mitochondrial reporter (mt-COX8-GFP) mice, wild-type (WT) mice, and Nrf2⁻/⁻ mice. Bronchoalveolar lavage, wet/dry ratios, real-time RT-PCR and Western analysis, immunohistochemistry, and fluorescence microscopy were performed on the lung at 0, 6, 24, and 48 h. The mice survived S. aureus inoculations at 5×10⁸ CFU despite diffuse lung inflammation and edema, but the Nrf2⁻/⁻ lung showed increased ALI. In mt-COX8-GFP mice, mitochondrial fluorescence was enhanced in bronchial and alveolar type II (AT2) epithelial cells. WT mice displayed rapid HO-1 upregulation and lower proinflammatory TNF-α, IL-1β, and CCL2 and, especially in AT2 cells, higher anti-inflammatory IL-10 and suppressor of cytokine signaling-3 than Nrf2⁻/⁻ mice. In the alveolar region, WT but not Nrf2⁻/⁻ mice showed strongly induced nuclear respiratory factor-1, PGC-1α, mitochondrial transcription factor-A, SOD2, Bnip3, mtDNA copy number, and citrate synthase. These findings indicate that S. aureus pneumonia induces Nrf2-dependent mitochondrial biogenesis in the alveolar region, mainly in AT2 cells. Absence of Nrf2 suppresses the alveolar transcriptional network for mitochondrial biogenesis and anti-inflammation, which worsens ALI. The findings link redox activation of mitochondrial biogenesis to ALI resolution.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Kollef MH, Micek ST. Staphylococcus aureus pneumonia: a superbug infection in community and hospital settings. Chest. 2005;128:1093–1097. - PubMed
-
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am. J. Respir. Crit. Care Med. 2003;167:1027–1035. - PubMed
-
- Demling RH. The modern version of adult respiratory distress syndrome. Annu. Rev. Med. 1995;46:193–202. - PubMed
-
- Fullerton JN, Singer M. Organ failure in the ICU: cellular alterations. Semin. Respir. Crit. Care Med. 2011;32:581–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

