Drosophila egg chamber elongation: insights into how tissues and organs are shaped
- PMID: 22940759
- PMCID: PMC3519655
- DOI: 10.4161/fly.21969
Drosophila egg chamber elongation: insights into how tissues and organs are shaped
Abstract
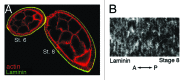
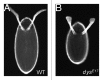
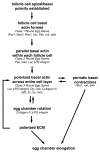
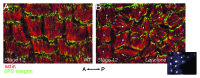
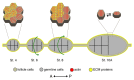
As tissues and organs are formed, they acquire a specific shape that plays an integral role in their ability to function properly. A relatively simple system that has been used to examine how tissues and organs are shaped is the formation of an elongated Drosophila egg. While it has been known for some time that Drosophila egg elongation requires interactions between a polarized intracellular basal actin network and a polarized extracellular network of basal lamina proteins, how these interactions contribute to egg elongation remained unclear. Recent studies using live imaging have revealed two novel processes, global tissue rotation and oscillating basal actomyosin contractions, which have provided significant insight into how the two polarized protein networks cooperate to produce an elongated egg. This review summarizes the proteins involved in Drosophila egg elongation and how this recent work has contributed to our current understanding of how egg elongation is achieved.
Figures

References
-
- Spradling A. Developmental Genetics of Oogenesis. In: Bate M, Martinez-Arias A, eds. The Development of Drosophila melanogaster Plainview, NY: Cold Spring Harbor Laboratory Press, 1993:1-70.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
