Retinoic acid and hydrocortisone strengthen the barrier function of human RPMI 2650 cells, a model for nasal epithelial permeability
- PMID: 22940916
- PMCID: PMC3597180
- DOI: 10.1007/s10616-012-9493-7
Retinoic acid and hydrocortisone strengthen the barrier function of human RPMI 2650 cells, a model for nasal epithelial permeability
Abstract
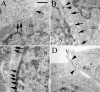
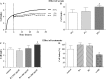
The nasal pathway represents an alternative route for non-invasive systemic administration of drugs. The main advantages of nasal drug delivery are the rapid onset of action, the avoidance of the first-pass metabolism in the liver and the easy applicability. In vitro cell culture systems offer an opportunity to model biological barriers. Our aim was to develop and characterize an in vitro model based on confluent layers of the human RPMI 2650 cell line. Retinoic acid, hydrocortisone and cyclic adenosine monophosphate, which influence cell attachment, growth and differentiation have been investigated on the barrier formation and function of the nasal epithelial cell layers. Real-time cell microelectronic sensing, a novel label-free technique was used for dynamic monitoring of cell growth and barrier properties of RPMI 2650 cells. Treatments enhanced the formation of adherens and tight intercellular junctions visualized by electron microscopy, the presence and localization of junctional proteins ZO-1 and β-catenin demonstrated by fluorescent immunohistochemistry, and the barrier function of nasal epithelial cell layers. The transepithelial resistance of the RPMI 2650 cell model reached 50 to 200 Ω × cm(2), the permeability coefficient for 4.4 kDa FITC-dextran was 9.3 to 17 × 10(-6) cm/s, in agreement with values measured on nasal mucosa from in vivo and ex vivo experiments. Based on these results human RPMI 2650 cells seem to be a suitable nasal epithelial model to test different pharmaceutical excipients and various novel formulations, such as nanoparticles for toxicity and permeability.
Figures


Similar articles
-
Comparison of RPMI 2650 cell layers and excised sheep nasal epithelial tissues in terms of nasal drug delivery and immunocytochemistry properties.J Pharmacol Toxicol Methods. 2022 Jan-Feb;113:107131. doi: 10.1016/j.vascn.2021.107131. Epub 2021 Oct 24. J Pharmacol Toxicol Methods. 2022. PMID: 34699972
-
Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs.Int J Pharm. 2016 Dec 30;515(1-2):1-10. doi: 10.1016/j.ijpharm.2016.09.086. Epub 2016 Oct 1. Int J Pharm. 2016. PMID: 27702697
-
Evaluation of human nasal RPMI 2650 cells grown at an air-liquid interface as a model for nasal drug transport studies.J Pharm Sci. 2008 Mar;97(3):1165-78. doi: 10.1002/jps.21031. J Pharm Sci. 2008. PMID: 17628494
-
Is RPMI 2650 a Suitable In Vitro Nasal Model for Drug Transport Studies?Eur J Drug Metab Pharmacokinet. 2018 Feb;43(1):13-24. doi: 10.1007/s13318-017-0426-x. Eur J Drug Metab Pharmacokinet. 2018. PMID: 28688000 Review.
-
The blood-brain and gut-vascular barriers: from the perspective of claudins.Tissue Barriers. 2021 Jul 3;9(3):1926190. doi: 10.1080/21688370.2021.1926190. Epub 2021 Jun 21. Tissue Barriers. 2021. PMID: 34152937 Free PMC article. Review.
Cited by
-
Optimization of an oral mucosa in vitro model based on cell line TR146.Tissue Barriers. 2020 Apr 2;8(2):1748459. doi: 10.1080/21688370.2020.1748459. Epub 2020 Apr 21. Tissue Barriers. 2020. PMID: 32314665 Free PMC article.
-
Edaravone protects against methylglyoxal-induced barrier damage in human brain endothelial cells.PLoS One. 2014 Jul 17;9(7):e100152. doi: 10.1371/journal.pone.0100152. eCollection 2014. PLoS One. 2014. PMID: 25033388 Free PMC article.
-
Development and Characterization of a Primary Ciliated Porcine Airway Model for the Evaluation of In Vitro Mucociliary Clearance and Mucosal Drug Delivery.Pharmaceutics. 2025 Apr 2;17(4):462. doi: 10.3390/pharmaceutics17040462. Pharmaceutics. 2025. PMID: 40284456 Free PMC article.
-
Alpha-Melanocyte Stimulating Hormone Protects against Cytokine-Induced Barrier Damage in Caco-2 Intestinal Epithelial Monolayers.PLoS One. 2017 Jan 19;12(1):e0170537. doi: 10.1371/journal.pone.0170537. eCollection 2017. PLoS One. 2017. PMID: 28103316 Free PMC article.
-
Characterization of critical parameters using an air-liquid interface model with RPMI 2650 cells for permeability studies of small molecules.Drug Deliv Transl Res. 2024 Jun;14(6):1601-1615. doi: 10.1007/s13346-023-01474-w. Epub 2023 Nov 17. Drug Deliv Transl Res. 2024. PMID: 37978162
References
-
- Agu RU, Jorissen M, Willems T, Augustijns P, Kinget R, Verbeke N. In vitro nasal drug delivery studies: comparison of derivatised, fibrillar and polymerised collagen matrix-based human nasal primary culture systems for nasal drug delivery studies. J Pharm Pharmacol. 2001;53:1447–1456. doi: 10.1211/0022357011777981. - DOI - PubMed
LinkOut - more resources
Full Text Sources

