Characterization and two-dimensional crystallization of membrane component AlkB of the medium-chain alkane hydroxylase system from Pseudomonas putida GPo1
- PMID: 22941083
- PMCID: PMC3485972
- DOI: 10.1128/AEM.02053-12
Characterization and two-dimensional crystallization of membrane component AlkB of the medium-chain alkane hydroxylase system from Pseudomonas putida GPo1
Abstract
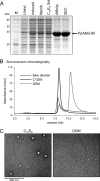
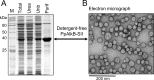
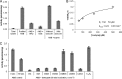
The alkane hydroxylase system of Pseudomonas putida GPo1 allows it to use alkanes as the sole source of carbon and energy. Bacterial alkane hydroxylases have tremendous potential as biocatalysts for the stereo- and regioselective transformation of a wide range of chemically inert unreactive alkanes into valuable reactive chemical precursors. We have produced and characterized the first 2-dimensional crystals of the integral membrane component of the P. putida alkane hydroxylase system, the nonheme di-iron alkane monooxygenase AlkB. Our analysis reveals for the first time that AlkB reconstituted into a lipid bilayer forms trimers. Addition of detergents that do not disrupt the AlkB oligomeric state (decyl maltose neopentyl glycol [DMNG], lauryl maltose neopentyl glycol [LMNG], and octaethylene glycol monododecyl ether [C(12)E(8)]) preserved its activity at a level close to that of the detergent-free control sample. In contrast, the monomeric form of AlkB produced by purification in n-decyl-β-D-maltopyranoside (DM), n-dodecyl-β-D-maltopyranoside (DDM), octyl glucose neopentyl glycol (OGNG), and n-dodecyl-N,N-dimethylamine-N-oxide (LDAO) was largely inactive. This is the first indication that the physiologically active form of membrane-embedded AlkB may be a multimer. We present for the first time experimental evidence that 1-octyne acts as a mechanism-based inhibitor of AlkB. Therefore, despite the lack of any significant full-length sequence similarity with members of other monooxygenase classes that catalyze the terminal oxidation of alkanes, AlkB is likely to share a similar catalytic mechanism.
Figures



References
-
- Ayala M, Torres E. 2004. Enzymatic activation of alkanes: constraints and prospective. Appl. Catal. A 272: 1– 13
-
- Cuff JA, Barton GJ. 2000. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins 40: 502– 511 - PubMed
-
- Eggink G, Lageveen RG, Altenburg B, Witholt B. 1987. Controlled and functional expression of the Pseudomonas oleovorans alkane utilizing system in Pseudomonas putida and Escherichia coli. J. Biol. Chem. 262: 17712– 17718 - PubMed
-
- Gipson B, Zeng X, Zhang ZY, Stahlberg H. 2007. 2dx—user-friendly image processing for 2D crystals. J. Struct. Biol. 157: 64– 72 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

