Biasing the content of hippocampal replay during sleep
- PMID: 22941111
- PMCID: PMC4354843
- DOI: 10.1038/nn.3203
Biasing the content of hippocampal replay during sleep
Abstract
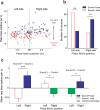
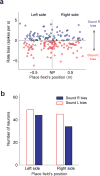
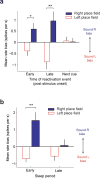
The hippocampus is essential for encoding self-experienced events into memory. During sleep, neural activity in the hippocampus related to a recent experience has been observed to spontaneously reoccur, and this 'replay' has been postulated to be important for memory consolidation. Task-related cues can enhance memory consolidation when presented during a post-training sleep session, and, if memories are consolidated by hippocampal replay, a specific enhancement for this replay should be observed. To test this, we trained rats on an auditory-spatial association task while recording from neuronal ensembles in the hippocampus. We found that, during sleep, a task-related auditory cue biased reactivation events toward replaying the spatial memory associated with that cue. These results indicate that sleep replay can be manipulated by external stimulation and provide further evidence for the role of hippocampal replay in memory consolidation.
Conflict of interest statement
The authors do not have any competing interests
Figures




Comment in
-
Sleep tight, wake up bright.Nat Neurosci. 2012 Oct;15(10):1327-9. doi: 10.1038/nn.3227. Nat Neurosci. 2012. PMID: 23007185 No abstract available.
References
References for Online Methods
-
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–7. - PubMed
-
- Zhang K, Ginzburg I, McNaughton BL, Sejnowski TJ. Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J Neurophysiol. 1998;79(2):1017–44. - PubMed
References
-
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. - PubMed
-
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11(10):442–50. - PubMed
-
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

