Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis
- PMID: 22941226
- PMCID: PMC3535352
- DOI: 10.1007/s00401-012-1039-8
Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis
Abstract
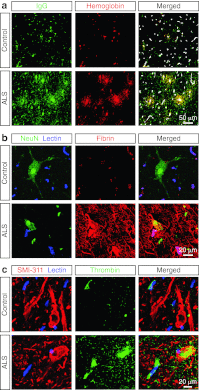
The blood-brain barrier and blood-spinal cord barrier (BSCB) limit the entry of plasma components and erythrocytes into the central nervous system (CNS). Pericytes play a key role in maintaining blood-CNS barriers. The BSCB is damaged in patients with amyotrophic lateral sclerosis (ALS). Moreover, transgenic ALS rodents and pericyte-deficient mice develop BSCB disruption with erythrocyte extravasation preceding motor neuron dysfunction. Here, we studied whether BSCB disruption with erythrocyte extravasation and pericyte loss are present in human ALS. We show that 11 of 11 cervical cords from ALS patients, but 0 of 5 non-neurodegenerative disorders controls, possess perivascular deposits of erythrocyte-derived hemoglobin and hemosiderin typically 10-50 μm in diameter suggestive of erythrocyte extravasation. Immunostaining for CD235a, a specific marker for erythrocytes, confirmed sporadic erythrocyte extravasation in ALS, but not controls. Quantitative analysis revealed a 3.1-fold increase in perivascular hemoglobin deposits in ALS compared to controls showing hemoglobin confined within the vascular lumen, which correlated with 2.5-fold increase in hemosiderin deposits (r = 0.82, p < 0.01). Spinal cord parenchymal accumulation of plasma-derived immunoglobulin G, fibrin and thrombin was demonstrated in ALS, but not controls. Immunostaining for platelet-derived growth factor receptor-β, a specific marker for CNS pericytes, indicated a 54 % (p < 0.01) reduction in pericyte number in ALS patients compared to controls. Pericyte reduction correlated negatively with the magnitude of BSCB damage as determined by hemoglobin abundance (r = -0.75, p < 0.01). Thus, the BSCB disruption with erythrocyte extravasation and pericyte reductions is present in ALS. Whether similar findings occur in motor cortex and affected brainstem motor nuclei remain to be seen.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

