Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss
- PMID: 22941241
- PMCID: PMC3473167
- DOI: 10.1007/s00401-012-1040-2
Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss
Abstract
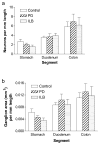
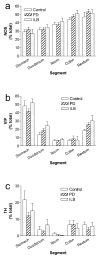
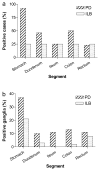
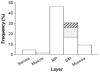
Gastrointestinal dysfunction is a prominent non-motor feature of Parkinson's disease (PD) that contributes directly to the morbidity of patients, complicates management of motor symptoms, and may herald incipient PD in patients without motor disability. Although PD has traditionally been considered a disease of dopaminergic neurons in the substantia nigra, analyses of gastrointestinal samples from PD patients have consistently revealed pathology in the enteric nervous system. The relationship of PD pathology to GI dysmotility is poorly understood, and this lack of understanding has led to limited success in developing treatments for PD-related GI symptoms. We have quantitatively compared myenteric neuron density and relative abundance of NO, VIP, and catecholamine neurons between patients with PD and control individuals along the length of the GI tract. In addition, we have examined the frequency of GI α-synuclein neuritic pathology and its co-localization with the same neuronal markers. We have included a comparison with a small population of patients with incidental Lewy bodies found at autopsy. These data indicate that there is no neuronal loss in the myenteric plexus in PD. Lewy body pathology parallels parasympathetic autonomic input from the dorsal motor nucleus of the vagus, not the distribution of extrinsic sympathetic input or intrinsic enteric neurons, and is only rarely co-localized with tyrosine hydroxylase. These data provide a critical background to which further analyses of the effect of PD on the GI tract may be compared and suggest that neuropathology in myenteric neurons is unlikely to be a causative factor in PD-related GI dysmotility.
Figures



References
-
- Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57 (3):456–462. - PubMed
-
- Anlauf M, Schafer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. The Journal of comparative neurology. 2003;459 (1):90–111. - PubMed
-
- Ashraf W, Wszolek ZK, Pfeiffer RF, Normand M, Maurer K, Srb F, Edwards LL, Quigley EM. Anorectal function in fluctuating (on-off) Parkinson’s disease: evaluation by combined anorectal manometry and electromyography. Mov Disord. 1995;10 (5):650–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

