Proteoglycan synthesis and Golgi organization in polarized epithelial cells
- PMID: 22941419
- PMCID: PMC3527882
- DOI: 10.1369/0022155412461256
Proteoglycan synthesis and Golgi organization in polarized epithelial cells
Abstract
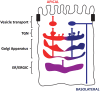
A large number of complex glycosylation mechanisms take place in the Golgi apparatus. In epithelial cells, glycosylated protein molecules are transported to both the apical and the basolateral surface domains. Although the prevailing view is that the Golgi apparatus provides the same lumenal environment for glycosylation of apical and basolateral cargo proteins, there are indications that proteoglycans destined for the two opposite epithelial surfaces are exposed to different conditions in transit through the Golgi apparatus. We will here review data relating proteoglycan and glycoprotein synthesis to characteristics of the apical and basolateral secretory pathways in epithelial cells.
Conflict of interest statement
Figures

References
-
- Almeida R, Levery SB, Mandel U, Kresse H, Schwientek T, Bennett EP, Clausen H. 1999. Cloning and expression of a proteoglycan UDP-galactose:beta-xylose beta1,4-galactosyltransferase I: A seventh member of the human beta4-galactosyltransferase gene family. J Biol Chem. 274:26165–26171 - PubMed
-
- Appenzeller-Herzog C, Roche AC, Nufer O, Hauri HP. 2004. pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. J Biol Chem. 279:12943–12950 - PubMed
-
- Ashikov A, Routier F, Fuhlrott J, Helmus Y, Wild M, Gerardy-Schahn R, Bakker H. 2005. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J Biol Chem. 280:27230–27235 - PubMed
-
- Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, Hansson GC. 2001. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 11:633–644 - PubMed
-
- Bai X, Zhou D, Brown JR, Crawford BE, Hennet T, Esko JD. 2001. Biosynthesis of the linkage region of glycosaminoglycans: cloning and activity of galactosyltransferase II, the sixth member of the beta 1,3-galactosyltransferase family (beta 3GalT6). J Biol Chem. 276:48189–48195 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

