PALB2 self-interaction controls homologous recombination
- PMID: 22941656
- PMCID: PMC3488246
- DOI: 10.1093/nar/gks807
PALB2 self-interaction controls homologous recombination
Abstract
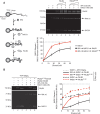
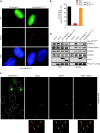
PALB2 is essential for BRCA2 anchorage to nuclear structures and for homologous recombinational repair of DNA double-strand breaks. Here, we report that the N-terminal coiled-coil motif of PALB2 regulates its self-association and homologous recombination. Monomeric PALB2 shows higher efficiency to bind DNA and promotes RAD51 filament formation with or without the inhibitory effect of Replication Protein A. Moreover, overexpression of the PALB2 coiled-coil domain severely affects RAD51 loading to DNA damage sites suggesting a competition between PALB2 self-interaction and PALB2-BRCA1 interaction. In the presence of DNA damage, the switch between PALB2-PALB2 and PALB2-BRCA1 interactions allows the activation of HR. Controlling HR via PALB2 self-interactions could be important to prevent aberrant recombination in normal conditions and activate DNA repair when required.
Figures




References
-
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Ann. Rev. Biochem. 2008;77:229–257. - PubMed
-
- Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, West SC. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell. 2001;7:273–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

