Effects of lipoic acid on lipolysis in 3T3-L1 adipocytes
- PMID: 22941773
- PMCID: PMC3465999
- DOI: 10.1194/jlr.M027086
Effects of lipoic acid on lipolysis in 3T3-L1 adipocytes
Abstract
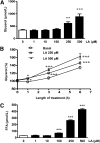
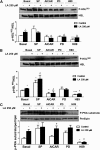
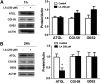
Lipoic acid (LA) is a naturally occurring compound with beneficial effects on obesity. The aim of this study was to evaluate its effects on lipolysis in 3T3-L1 adipocytes and the mechanisms involved. Our results revealed that LA induced a dose- and time-dependent lipolytic action, which was reversed by pretreatment with the c-Jun N-terminal kinase inhibitor SP600125, the PKA inhibitor H89, and the AMP-activated protein kinase activator AICAR. In contrast, the PI3K/Akt inhibitor LY294002 and the PDE3B antagonist cilostamide enhanced LA-induced lipolysis. LA treatment for 1 h did not modify total protein content of hormone-sensitive lipase (HSL) but significantly increased the phosphorylation of HSL at Ser(563) and at Ser(660), which was reversed by H89. LA treatment also induced a marked increase in PKA-mediated perilipin phosphorylation. LA did not significantly modify the protein levels of adipose triglyceride lipase or its activator comparative gene identification 58 (CGI-58) and inhibitor G(0)/G(1) switch gene 2 (G0S2). Furthermore, LA caused a significant inhibition of adipose-specific phospholipase A2 (AdPLA) protein and mRNA levels in parallel with a decrease in the amount of prostaglandin E(2) released and an increase in cAMP content. Together, these data suggest that the lipolytic actions of LA are mainly mediated by phosphorylation of HSL through cAMP-mediated activation of protein kinase A probably through the inhibition of AdPLA and prostaglandin E(2).
Figures



Similar articles
-
Cardiotrophin-1 stimulates lipolysis through the regulation of main adipose tissue lipases.J Lipid Res. 2014 Dec;55(12):2634-43. doi: 10.1194/jlr.M055335. Epub 2014 Oct 28. J Lipid Res. 2014. PMID: 25351614 Free PMC article.
-
Lipoic acid inhibits adiponectin production in 3T3-L1 adipocytes.J Physiol Biochem. 2013 Sep;69(3):595-600. doi: 10.1007/s13105-012-0230-7. Epub 2013 Jan 12. J Physiol Biochem. 2013. PMID: 23307774
-
Extract of Paecilomyces hepiali mycelia induces lipolysis through PKA-mediated phosphorylation of hormone-sensitive lipase and ERK-mediated downregulation of perilipin in 3T3-L1 adipocytes.BMC Complement Altern Med. 2018 Dec 7;18(1):326. doi: 10.1186/s12906-018-2389-0. BMC Complement Altern Med. 2018. PMID: 30526586 Free PMC article.
-
Serum amyloid A induces lipolysis by downregulating perilipin through ERK1/2 and PKA signaling pathways.Obesity (Silver Spring). 2011 Dec;19(12):2301-9. doi: 10.1038/oby.2011.176. Epub 2011 Jun 23. Obesity (Silver Spring). 2011. PMID: 21701568
-
Regulation of adipocyte lipolysis.Nutr Res Rev. 2014 Jun;27(1):63-93. doi: 10.1017/S095442241400002X. Epub 2014 May 28. Nutr Res Rev. 2014. PMID: 24872083 Review.
Cited by
-
Zyflamend, a unique herbal blend, induces cell death and inhibits adipogenesis through the coordinated regulation of PKA and JNK.Adipocyte. 2020 Dec;9(1):454-471. doi: 10.1080/21623945.2020.1803642. Adipocyte. 2020. PMID: 32779962 Free PMC article.
-
Nutritional factors affecting abdominal fat deposition in poultry: a review.Asian-Australas J Anim Sci. 2014 Jul;27(7):1057-68. doi: 10.5713/ajas.2013.13702. Asian-Australas J Anim Sci. 2014. PMID: 25050050 Free PMC article.
-
Sudachitin and Nobiletin Stimulate Lipolysis via Activation of the cAMP/PKA/HSL Pathway in 3T3-L1 Adipocytes.Foods. 2023 May 10;12(10):1947. doi: 10.3390/foods12101947. Foods. 2023. PMID: 37238764 Free PMC article.
-
Limited oxygen in standard cell culture alters metabolism and function of differentiated cells.EMBO J. 2024 Jun;43(11):2127-2165. doi: 10.1038/s44318-024-00084-7. Epub 2024 Apr 5. EMBO J. 2024. PMID: 38580776 Free PMC article.
-
Activation of AMPKα2 in adipocytes is essential for nicotine-induced insulin resistance in vivo.Nat Med. 2015 Apr;21(4):373-82. doi: 10.1038/nm.3826. Epub 2015 Mar 23. Nat Med. 2015. PMID: 25799226 Free PMC article.
References
-
- Scholich H., Murphy M. E., Sies H. 1989. Antioxidant activity of dihydrolipoate against microsomal lipid peroxidation and its dependence on alpha-tocopherol. Biochim. Biophys. Acta. 1001: 256–261 - PubMed
-
- Han D., Tritschler H. J., Packer L. 1995. Alpha-lipoic acid increases intracellular glutathione in a human T-lymphocyte Jurkat cell line. Biochem. Biophys. Res. Commun. 207: 258–264 - PubMed
-
- Kim M. S., Park J. Y., Namkoong C., Jang P. G., Ryu J. W., Song H. S., Yun J. Y., Namgoong I. S., Ha J., Park I. S., et al. 2004. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 10: 727–733 - PubMed
-
- Prieto-Hontoria P. L., Perez-Matute P., Fernandez-Galilea M., Barber A., Martinez J. A., Moreno-Aliaga M. J. 2009. Lipoic acid prevents body weight gain induced by a high fat diet in rats: effects on intestinal sugar transport. J. Physiol. Biochem. 65: 43–50 - PubMed
-
- Carbonelli M. G., Di Renzo L., Bigioni M., Di Daniele N., De Lorenzo A., Fusco M. A. 2010. Alpha-lipoic acid supplementation: a tool for obesity therapy? Curr. Pharm. Des. 16: 840–846 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

