Protonation state of E71 in KcsA and its role for channel collapse and inactivation
- PMID: 22942391
- PMCID: PMC3458351
- DOI: 10.1073/pnas.1211900109
Protonation state of E71 in KcsA and its role for channel collapse and inactivation
Abstract
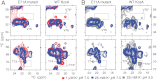
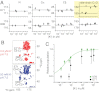
The prototypical prokaryotic potassium channel KcsA alters its pore depending on the ambient potassium; at high potassium, it exists in a conductive form, and at low potassium, it collapses into a nonconductive structure with reduced ion occupancy. We present solid-state NMR studies of KcsA in which we test the hypothesis that an important channel-inactivation process, known as C-type inactivation, proceeds via a state similar to this collapsed state. We test this using an inactivation-resistant mutant E71A, and show that E71A is unable to collapse its pore at both low potassium and low pH, suggesting that the collapsed state is structurally similar to the inactivated state. We also show that E71A has a disordered selectivity filter. Using site-specific K(+) titrations, we detect a local change at E71 that is coupled to channel collapse at low K(+). To gain more insight into this change, we site specifically measure the chemical shift tensors of the side-chain carboxyls of E71 and its hydrogen bond partner D80, and use the tensors to assign protonation states to E71 and D80 at high K(+) and neutral pH. Our measurements show that E71 is protonated at pH 7.5 and must have an unusually perturbed pK(a) (> 7.5) suggesting that the change at E71 is a structural rearrangement rather than a protonation event. The results offer new mechanistic insights into why the widely used mutant KcsA-E71A does not inactivate and establish the ambient K(+) level as a means to populate the inactivated state of KcsA in a controlled way.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. - PubMed
-
- Bowlby M, Peri R, Zhang H, Dunlop J. hERG (KCNH2 or Kv11.1) K+ channels: Screening for cardiac arrhythmia risk. Curr Drug Metab. 2008;9:965–970. - PubMed
-
- Zhou Y, Morais-Cabral JH, Kaufman A, Mackinnon R. Chemistry of ion coordination and hydration revealed by a K channel Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. - PubMed
-
- Cordero-Morales JF, Cuello LG, Perozo E. Voltage-dependent gating at the KcsA selectivity filter. Nat Struct Mol Biol. 2006;13:319–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

