Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice
- PMID: 22942425
- PMCID: PMC3448973
- DOI: 10.4049/jimmunol.1201216
Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice
Abstract
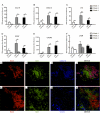
Salivary glands in patients with Sjögren's syndrome (SS) develop ectopic lymphoid structures (ELS) characterized by B/T cell compartmentalization, the formation of high endothelial venules, follicular dendritic cell networks, functional B cell activation with expression of activation-induced cytidine deaminase, as well as local differentiation of autoreactive plasma cells. The mechanisms that trigger ELS formation, autoimmunity, and exocrine dysfunction in SS are largely unknown. In this article, we present a novel model of inducible ectopic lymphoid tissue formation, breach of humoral self-tolerance, and salivary hypofunction after delivery of a replication-deficient adenovirus-5 in submandibular glands of C57BL/6 mice through retrograde excretory duct cannulation. In this model, inflammation rapidly and consistently evolves from diffuse infiltration toward the development of SS-like periductal lymphoid aggregates within 2 wk from AdV delivery. These infiltrates progressively acquire ELS features and support functional GL7(+)/activation-induced cytidine deaminase(+) germinal centers. Formation of ELS is preceded by ectopic expression of lymphoid chemokines CXCL13, CCL19, and lymphotoxin-β, and is associated with development of anti-nuclear Abs in up to 75% of mice. Finally, reduction in salivary flow was observed over 3 wk post-AdV infection, consistent with exocrine gland dysfunction as a consequence of the inflammatory response. This novel model has the potential to unravel the cellular and molecular mechanisms that regulate ELS formation and their role in exocrine dysfunction and autoimmunity in SS.
Figures




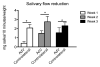
References
-
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. - PubMed
-
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. - PubMed
-
- Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev. 2010;233:267–285. - PubMed
-
- Routsias JG, Tzioufas AG, Moutsopoulos HM. The clinical value of intracellular autoantigens B-cell epitopes in systemic rheumatic diseases. Clin Chim Acta. 2004;340:1–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

