Cyanovirin-N inhibits mannose-dependent Mycobacterium-C-type lectin interactions but does not protect against murine tuberculosis
- PMID: 22942435
- PMCID: PMC3448799
- DOI: 10.4049/jimmunol.1102408
Cyanovirin-N inhibits mannose-dependent Mycobacterium-C-type lectin interactions but does not protect against murine tuberculosis
Abstract
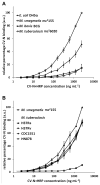
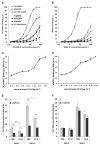
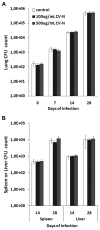
Cyanovirin-N (CV-N) is a mannose-binding lectin that inhibits HIV-1 infection by blocking mannose-dependent target cell entry via C-type lectins. Like HIV-1, Mycobacterium tuberculosis expresses mannosylated surface structures and exploits C-type lectins to gain cell access. In this study, we investigated whether CV-N, like HIV-1, can inhibit M. tuberculosis infection. We found that CV-N specifically interacted with mycobacteria by binding to the mannose-capped lipoglycan lipoarabinomannan. Furthermore, CV-N competed with the C-type lectins DC-SIGN and mannose receptor for ligand binding and inhibited the binding of M. tuberculosis to dendritic cells but, unexpectedly, not to macrophages. Subsequent in vivo infection experiments in a mouse model demonstrated that, despite its activity, CV-N did not inhibit or delay M. tuberculosis infection. This outcome argues against a critical role for mannose-dependent C-type lectin interactions during the initial stages of murine M. tuberculosis infection and suggests that, depending on the circumstances, M. tuberculosis can productively infect cells using different modes of entry.
Conflict of interest statement
The authors declare that no conflict of interest exists related to this work.
Figures

References
-
- World Health Organization. Global tuberculosis control: WHO report 2010. WHO Press; Switzerland: 2010. Report No.: WHO/HTM/TB/2010.7.
-
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. - PubMed
-
- Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

