Lactate as a biomarker for sleep
- PMID: 22942499
- PMCID: PMC3413798
- DOI: 10.5665/sleep.2072
Lactate as a biomarker for sleep
Abstract
Study objectives: An ideal biomarker for sleep should change rapidly with sleep onset, remain at a detectably differential level throughout the sleep period, and exhibit a rapid change with waking. Currently, no molecular marker has been identified that exhibits all three properties. This study examined three substances (lactate, glucose, and glutamate) for suitability as a sleep biomarker.
Design: Using amperometric biosensor technology in conjunction with electroencephalograph (EEG) and electromyograph (EMG) monitoring, extracellular concentrations of lactate and glucose (Cohort 1) as well as lactate and glutamate (Cohort 2) were recorded over multiple sleep/wake cycles.
Patients or participants: There were 12 C57Bl/6J male mice (3-5 mo old).
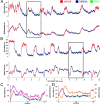
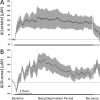
Interventions: Sleep and waking transitions were identified using EEG recordings. Extracellular concentrations of lactate, glucose, and glutamate were evaluated before and during transition events as well as during extended sleep and during a 6-h sleep deprivation period.
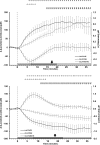
Measurements and results: Rapid and sustained increases in cortical lactate concentration (approximately 15 μM/min) were immediately observed upon waking and during rapid eye movement sleep. Elevated lactate concentration was also maintained throughout a 6-h period of continuous waking. A persistent and sustained decline in lactate concentration was measured during nonrapid eye movement sleep. Glutamate exhibited similar patterns, but with a much slower rise and decline (approximately 0.03 μM/min). Glucose concentration changes did not demonstrate a clear correlation with either sleep or wake.
Conclusions: These findings indicate that extracellular lactate concentration is a reliable sleep/wake biomarker and can be used independently of the EEG signal.
Keywords: Biosensor; continuous in vivo monitoring; electroencephalography; electromyography; glucose; glutamate; lactate; mouse; sleep.
Figures




References
-
- Netchiporouk L, Shram N, Salvert D, Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur. J. Neurosci. 2001;13:1429–34. - PubMed
-
- Shram N, Netchiporouk L, Cespuglio R. Lactate in the brain of the freely moving rat: voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci. 2002;16:461–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

