Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine
- PMID: 22942642
- PMCID: PMC3428248
- DOI: 10.2147/IJN.S34991
Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine
Abstract
Purpose: To develop nanostructured-lipid carriers (NLCs) coated with cell-penetrating peptides (CPP) for improving the oral bioavailability of tripterine.
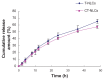
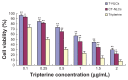
Methods: We prepared CPP-coated tripterine-loaded NLCs (CT-NLCs) by using a solvent evaporation method, and determined their physical properties. In vitro drug release was determined by using a dialysis bag diffusion technique, and intestinal toxicity was evaluated by performing MTT assay using Caco-2 cells. In vivo absorption was studied in an in situ rat intestinal perfusion model, and oral bioavailability was examined in beagles.
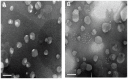
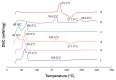
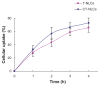
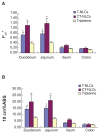
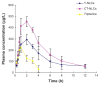
Results: The average particle size, zeta potential, and encapsulation efficiency of the optimized CT-NLCs were 126.7 ± 9.2 nm, 28.7 ± 3.4 mV, and 72.64% ± 1.37%, respectively. The CT-NLCs showed a controlled release profile in vitro and had significantly lower intestinal cytotoxicity than the tripterine solution (P < 0.05). The absorption levels of tripterine from the CT-NLCs in the rat duodenum and jejunum were markedly higher than with tripterine-loaded NLCs without the CPP coating (T-NLCs), and with tripterine solution. Pharmacokinetic study showed that the maximum concentration of the CT-NLCs was greater than that of the T-NLCs and tripterine suspension, and that the time to maximum concentration of the CT-NLCs as well as the T-NLCs, was longer than that of the tripterine suspension. The relative oral bioavailability of the CT-NLCs compared to that of tripterine suspension and T-NLCs were 484.75% and 149.91% respectively.
Conclusion: The oral bioavailability of tripterine is dramatically increased by CT-NLCs. Therefore, CT-NLCs seem to be a promising carrier for oral delivery of tripterine.
Keywords: bioavailability; cell-penetrating peptides; nanostructured lipid carriers; oral drug delivery; tripterine.
Figures
Similar articles
-
Preparation of tripterine nanostructured lipid carriers and their absorption in rat intestine.Pharmazie. 2012 Apr;67(4):304-10. Pharmazie. 2012. PMID: 22570936
-
Size-exclusive effect of nanostructured lipid carriers on oral drug delivery.Int J Pharm. 2016 Sep 10;511(1):524-537. doi: 10.1016/j.ijpharm.2016.07.049. Epub 2016 Jul 22. Int J Pharm. 2016. PMID: 27452421
-
Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers.Int J Nanomedicine. 2012;7:3023-32. doi: 10.2147/IJN.S32476. Epub 2012 Jun 19. Int J Nanomedicine. 2012. PMID: 22787398 Free PMC article.
-
Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration.Drug Deliv. 2015;22(6):691-700. doi: 10.3109/10717544.2014.898110. Epub 2014 Mar 27. Drug Deliv. 2015. PMID: 24670099 Review.
-
Edible pentacyclic triterpenes: A review of their sources, bioactivities, bioavailability, self-assembly behavior, and emerging applications as functional delivery vehicles.Crit Rev Food Sci Nutr. 2024;64(16):5203-5219. doi: 10.1080/10408398.2022.2153238. Epub 2022 Dec 8. Crit Rev Food Sci Nutr. 2024. PMID: 36476115 Review.
Cited by
-
Nanoformulation of Leonotis leonurus to improve its bioavailability as a potential antidiabetic drug.3 Biotech. 2017 Oct;7(5):344. doi: 10.1007/s13205-017-0986-0. Epub 2017 Sep 23. 3 Biotech. 2017. PMID: 28955641 Free PMC article.
-
Approaches for enhancing oral bioavailability of peptides and proteins.Int J Pharm. 2013 Apr 15;447(1-2):75-93. doi: 10.1016/j.ijpharm.2013.02.030. Epub 2013 Feb 18. Int J Pharm. 2013. PMID: 23428883 Free PMC article. Review.
-
Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model.Int J Nanomedicine. 2013;8:4339-50. doi: 10.2147/IJN.S51621. Epub 2013 Nov 5. Int J Nanomedicine. 2013. PMID: 24235831 Free PMC article.
-
Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery.Heliyon. 2022 Feb 11;8(2):e08938. doi: 10.1016/j.heliyon.2022.e08938. eCollection 2022 Feb. Heliyon. 2022. PMID: 35198788 Free PMC article. Review.
-
Celastrol: An Update on Its Hepatoprotective Properties and the Linked Molecular Mechanisms.Front Pharmacol. 2022 Apr 4;13:857956. doi: 10.3389/fphar.2022.857956. eCollection 2022. Front Pharmacol. 2022. PMID: 35444532 Free PMC article. Review.
References
-
- Jang SY, Jang SW, Ko J. Celastrol inhibits the growth of estrogen positive human breast cancer cells through modulation of estrogen receptor α. Cancer Lett. 2011;300(1):57–65. - PubMed
-
- Ríos JL. Effects of triterpenes on the immune system. J Ethnopharmacol. 2010;128(1):1–14. - PubMed
-
- Kim DY, Park JW, Jeoung D, Ro JY. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur J Pharmacol. 2009;612(1–3):98–105. - PubMed
-
- Setty AR, Sigal LH. Herbal medications commonly used in the practice of rheumatology: mechanisms of action, efficacy, and side effects. Semin Arthritis Rheum. 2005;34(6):773–784. - PubMed
-
- Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303(1):9–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

