DsHsp90 is involved in the early response of Dunaliella salina to environmental stress
- PMID: 22942684
- PMCID: PMC3430215
- DOI: 10.3390/ijms13077963
DsHsp90 is involved in the early response of Dunaliella salina to environmental stress
Abstract
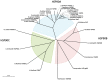
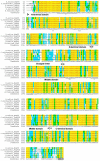
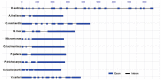
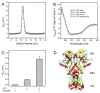
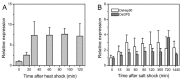
Heat shock protein 90 (Hsp90) is a molecular chaperone highly conserved across the species from prokaryotes to eukaryotes. Hsp90 is essential for cell viability under all growth conditions and is proposed to act as a hub of the signaling network and protein homeostasis of the eukaryotic cells. By interacting with various client proteins, Hsp90 is involved in diverse physiological processes such as signal transduction, cell mobility, heat shock response and osmotic stress response. In this research, we cloned the dshsp90 gene encoding a polypeptide composed of 696 amino acids from the halotolerant unicellular green algae Dunaliella salina. Sequence alignment indicated that DsHsp90 belonged to the cytosolic Hsp90A family. Further biophysical and biochemical studies of the recombinant protein revealed that DsHsp90 possessed ATPase activity and existed as a dimer with similar percentages of secondary structures to those well-studied Hsp90As. Analysis of the nucleotide sequence of the cloned genomic DNA fragment indicated that dshsp90 contained 21 exons interrupted by 20 introns, which is much more complicated than the other plant hsp90 genes. The promoter region of dshsp90 contained putative cis-acting stress responsive elements and binding sites of transcriptional factors that respond to heat shock and salt stress. Further experimental research confirmed that dshsp90 was upregulated quickly by heat and salt shock in the D. salina cells. These findings suggested that dshsp90 might serve as a component of the early response system of the D. salina cells against environmental stresses.
Keywords: Dunaliella salina; Hsp90; gene structure; haloadaption; heat shock; osmotic stress; structural feature.
Figures

Similar articles
-
Dunaliella salina Hsp90 is halotolerant.Int J Biol Macromol. 2015 Apr;75:418-25. doi: 10.1016/j.ijbiomac.2015.01.057. Epub 2015 Feb 11. Int J Biol Macromol. 2015. PMID: 25680963
-
Molecular characterization and expression of a gene encoding cytosolic Hsp90 from Pennisetum glaucum and its role in abiotic stress adaptation.Gene. 2011 Mar 15;474(1-2):29-38. doi: 10.1016/j.gene.2010.12.004. Epub 2010 Dec 22. Gene. 2011. PMID: 21185362
-
Functional implication of heat shock protein 70/90 and tubulin in cold stress of Dermacentor silvarum.Parasit Vectors. 2021 Oct 19;14(1):542. doi: 10.1186/s13071-021-05056-y. Parasit Vectors. 2021. PMID: 34666804 Free PMC article.
-
Mechanisms of Hsp90 regulation.Biochem J. 2016 Aug 15;473(16):2439-52. doi: 10.1042/BCJ20160005. Biochem J. 2016. PMID: 27515256 Free PMC article. Review.
-
The HSP90 complex of plants.Biochim Biophys Acta. 2012 Mar;1823(3):689-97. doi: 10.1016/j.bbamcr.2011.09.016. Epub 2011 Oct 6. Biochim Biophys Acta. 2012. PMID: 22001401 Review.
Cited by
-
The Carotenogenic Dunaliella salina CCAP 19/20 Produces Enhanced Levels of Carotenoid under Specific Nutrients Limitation.Biomed Res Int. 2018 Apr 30;2018:7532897. doi: 10.1155/2018/7532897. eCollection 2018. Biomed Res Int. 2018. PMID: 29854788 Free PMC article.
-
Osmotic Stress Induced Cell Death in Wheat Is Alleviated by Tauroursodeoxycholic Acid and Involves Endoplasmic Reticulum Stress-Related Gene Expression.Front Plant Sci. 2017 May 3;8:667. doi: 10.3389/fpls.2017.00667. eCollection 2017. Front Plant Sci. 2017. PMID: 28515732 Free PMC article.
-
Transcriptomic Characterization of Tambaqui (Colossoma macropomum, Cuvier, 1818) Exposed to Three Climate Change Scenarios.PLoS One. 2016 Mar 28;11(3):e0152366. doi: 10.1371/journal.pone.0152366. eCollection 2016. PLoS One. 2016. PMID: 27018790 Free PMC article.
-
Analysis of carotenogenic genes promoters and WRKY transcription factors in response to salt stress in Dunaliella bardawil.Sci Rep. 2017 Jan 27;7:37025. doi: 10.1038/srep37025. Sci Rep. 2017. PMID: 28128303 Free PMC article.
-
MdNup62 involved in salt and osmotic stress tolerance in apple.Sci Rep. 2023 Nov 18;13(1):20198. doi: 10.1038/s41598-023-47024-9. Sci Rep. 2023. PMID: 37980385 Free PMC article.
References
-
- Chen H., Jiang J.G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell Physiol. 2009;219:251–258. - PubMed
-
- Hosseini Tafreshi A., Shariati M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol. 2009;107:14–35. - PubMed
-
- Lamers P.P., Janssen M., de Vos R.C., Bino R.J., Wijffels R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008;26:631–638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

