The tricyclodecan-9-yl-xanthogenate D609 triggers ceramide increase and enhances FasL-induced caspase-dependent and -independent cell death in T lymphocytes
- PMID: 22942738
- PMCID: PMC3430269
- DOI: 10.3390/ijms13078834
The tricyclodecan-9-yl-xanthogenate D609 triggers ceramide increase and enhances FasL-induced caspase-dependent and -independent cell death in T lymphocytes
Abstract
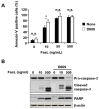
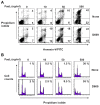
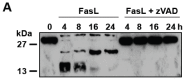
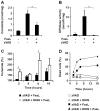
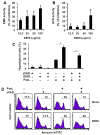
D609 is known to modulate death receptor-induced ceramide generation and cell death. We show that in Jurkat cells, non-toxic D609 concentrations inhibit sphingomyelin synthase and, to a lesser extent, glucosylceramide synthase, and transiently increase the intracellular ceramide level. D609 significantly enhanced FasL-induced caspase activation and apoptosis. D609 stimulated FasL-induced cell death in caspase-8-deficient Jurkat cells, indicating that D609 acts downstream of caspase-8. At high FasL concentration (500 ng/mL), cell death was significantly, but not completely, inhibited by zVAD-fmk, a broad-spectrum caspase inhibitor, indicating that FasL can activate both caspase-dependent and -independent cell death signaling pathways. FasL-induced caspase activation was abolished by zVAD-fmk, whereas ceramide production was only partially impaired. D609 enhanced caspase-independent ceramide increase and cell death in response to FasL. Also, D609 overcame zVAD-fmk-conferred resistance to a FasL concentration as low as 50 ng/mL and bypassed RIP deficiency. It is likely that mitochondrial events were involved, since Bcl-xL over-expression impaired D609 effects. In PHA-activated human T lymphocytes, D609 enhanced FasL-induced cell death in the presence or absence of zVAD-fmk. Altogether, our data strongly indicate that the inhibition of ceramide conversion to complex sphingolipids by D609 is accompanied by an enhancement of FasL-induced caspase-dependent and -independent cell death in T lymphocytes.
Keywords: ALPS; CD95; apoptosis; glucosylceramide synthase; necrosis; sphingomyelin synthase.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

