Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract
- PMID: 22942765
- PMCID: PMC3430296
- DOI: 10.3390/ijms13079260
Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract
Abstract
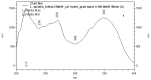
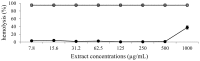
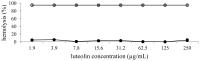
The biological activities of a plant extract depend on a complex sum of individual properties including the antioxidant activity. Several biological activities protect against the harmful action of reactive oxygen species (ROS), and here we focused our attention on the relationship between the biological activities tested and the antioxidant properties. In this study, the total flavonoid content as well as the antioxidant, antimicrobial, hemolytic and cytotoxicity activities of the methanolic extract of Leitothrix spiralis leaves were evaluated. The extract showed a total flavonoid content of 19.26% and the chemical characterization by HPLC-PAD confirmed the presence of flavonoids as the major secondary metabolite compounds. Significant antioxidant activity (IC(50) = 1.743 μg/mL ± 0.063) was demonstrated and was effective against Gram-negative organisms and all Candida strains tested, and showed an ability to inhibit hyphal formation. Non-hemolytic and antiproliferative activity could be demonstrated.
Keywords: Leiothrix spiralis; antimicrobial; antioxidant; citotoxicity; hemolytic; luteolin.
Figures






References
-
- Hu H., Zhang Z., Lei Z., Yang Y., Sugiura N. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J. Biosci. Bioeng. 2009;107:42–48. - PubMed
-
- Song J.M., Lee K.H., Seong B.L. Antiviral effect of catechins in green tea on influenza vírus. Antivir. Res. 2005;68:66–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

