Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system
- PMID: 22942910
- PMCID: PMC3430146
- DOI: 10.1002/wdev.52
Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system
Abstract
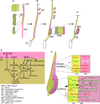
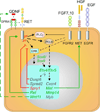
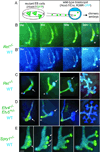
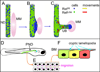
The mammalian kidney, which at maturity contains thousands of nephrons joined to a highly branched collecting duct (CD) system, is an important model system for studying the development of a complex organ. Furthermore, congenital anomalies of the kidney and urinary tract, often resulting from defects in ureteric bud branching morphogenesis, are relatively common human birth defects. Kidney development is initiated by interactions between the nephric duct and the metanephric mesenchyme, leading to the outgrowth and repeated branching of the ureteric bud epithelium, which gives rise to the entire renal CD system. Meanwhile, signals from the ureteric bud induce the mesenchyme cells to form the nephron epithelia. This review focuses on development of the CD system, with emphasis on the mouse as an experimental system. The major topics covered include the origin and development of the nephric duct, formation of the ureteric bud, branching morphogenesis of the ureteric bud, and elongation of the CDs. The signals, receptors, transcription factors, and other regulatory molecules implicated in these processes are discussed. In addition, our current knowledge of cellular behaviors that are controlled by these genes and underlie development of the collecting system is reviewed.
Figures


References
-
- Cebrian C, Borodo K, Charles N, Herzlinger DA. Morphometric index of the developing murine kidney. Dev Dyn. 2004;231:601–608. - PubMed
-
- Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. - PubMed
-
- Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. - PubMed
-
- Cullen-McEwen LA, Caruana G, Bertram JF. The where, what and why of the developing renal stroma. Nephron Exp Nephrol. 2005;99:e1–e8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

