Role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasis
- PMID: 22943001
- PMCID: PMC3430470
- DOI: 10.1002/cphy.c110024
Role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasis
Abstract
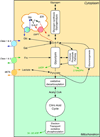
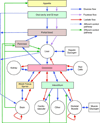
The facilitated diffusion of glucose, galactose, fructose, urate, myoinositol, and dehydroascorbicacid in mammals is catalyzed by a family of 14 monosaccharide transport proteins called GLUTs. These transporters may be divided into three classes according to sequence similarity and function/substrate specificity. GLUT1 appears to be highly expressed in glycolytically active cells and has been coopted in vitamin C auxotrophs to maintain the redox state of the blood through transport of dehydroascorbate. Several GLUTs are definitive glucose/galactose transporters, GLUT2 and GLUT5 are physiologically important fructose transporters, GLUT9 appears to be a urate transporter while GLUT13 is a proton/myoinositol cotransporter. The physiologic substrates of some GLUTs remain to be established. The GLUTs are expressed in a tissue specific manner where affinity, specificity, and capacity for substrate transport are paramount for tissue function. Although great strides have been made in characterizing GLUT-catalyzed monosaccharide transport and mapping GLUT membrane topography and determinants of substrate specificity, a unifying model for GLUT structure and function remains elusive. The GLUTs play a major role in carbohydrate homeostasis and the redistribution of sugar-derived carbons among the various organ systems. This is accomplished through a multiplicity of GLUT-dependent glucose sensing and effector mechanisms that regulate monosaccharide ingestion, absorption,distribution, cellular transport and metabolism, and recovery/retention. Glucose transport and metabolism have coevolved in mammals to support cerebral glucose utilization.
© 2012 American Physiological Society. Compr Physiol 2:933-996, 2012.
Figures



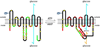
References
-
- Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. - PMC - PubMed
-
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. - PubMed
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. - PubMed
-
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature. 2004;427:803–807. - PubMed
-
- Adachi A. Electrophysiological study of hepatic vagal projection to the medulla. Neurosci Lett. 1981;24:19–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

