2-APB-potentiated channels amplify CatSper-induced Ca(2+) signals in human sperm
- PMID: 22943284
- PMCID: PMC3492921
- DOI: 10.1042/BJ20120339
2-APB-potentiated channels amplify CatSper-induced Ca(2+) signals in human sperm
Abstract
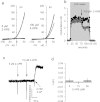
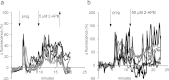
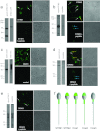
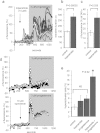
Ca2+i signalling is pivotal to sperm function. Progesterone, the best-characterized agonist of human sperm Ca2+i signalling, stimulates a biphasic [Ca2+]i rise, comprising a transient and subsequent sustained phase. In accordance with recent reports that progesterone directly activates CatSper, the [Ca2+]i transient was detectable in the anterior flagellum (where CatSper is expressed) 1-2 s before responses in the head and neck. Pre-treatment with 5 μM 2-APB (2-aminoethoxydiphenyl borate), which enhances activity of store-operated channel proteins (Orai) by facilitating interaction with their activator [STIM (stromal interaction molecule)] 'amplified' progesterone-induced [Ca2+]i transients at the sperm neck/midpiece without modifying kinetics. The flagellar [Ca2+]i response was unchanged. 2-APB (5 μM) also enhanced the sustained response in the midpiece, possibly reflecting mitochondrial Ca2+ accumulation downstream of the potentiated [Ca2+]i transient. Pre-treatment with 50-100 μM 2-APB failed to potentiate the transient and suppressed sustained [Ca2+]i elevation. When applied during the [Ca2+]i plateau, 50-100 μM 2-APB caused a transient fall in [Ca2+]i, which then recovered despite the continued presence of 2-APB. Loperamide (a chemically different store-operated channel agonist) enhanced the progesterone-induced [Ca2+]i signal and potentiated progesterone-induced hyperactivated motility. Neither 2-APB nor loperamide raised pHi (which would activate CatSper) and both compounds inhibited CatSper currents. STIM and Orai were detected and localized primarily to the neck/midpiece and acrosome where Ca2+ stores are present and the effects of 2-APB are focussed, but store-operated currents could not be detected in human sperm. We propose that 2-APB-sensitive channels amplify [Ca2+]i elevation induced by progesterone (and other CatSper agonists), amplifying, propagating and providing spatio-temporal complexity in [Ca2+]i signals of human sperm.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

