Antimicrobial activity of synthetic bornyl benzoates against Trypanosoma cruzi
- PMID: 22943546
- PMCID: PMC4001496
- DOI: 10.1179/2047773212Y.0000000002
Antimicrobial activity of synthetic bornyl benzoates against Trypanosoma cruzi
Abstract
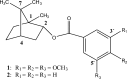
We report here for the first time the in vitro effects of (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2·2·1]heptan-2-yl-3',4',5'-trimethoxy benzoate (1) and (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2·2·1]heptan-2-yl benzoate (2) on the growth and ultrastructure of Trypanosoma cruzi. These two synthetic compounds exerted an antiproliferative effect on the epimastigote forms of the parasite. The ICs(50/72h) of two synthetic L-bornyl benzoates, 1 and 2, was 10·1 and 12·8 μg/ml, respectively. Both compounds were more selective against epimastigotes than HEp-2 cells. Ultrastructural analysis revealed intense cytoplasmic vacuolization and the appearance of cytoplasmic materials surrounded by membranes. The treatment of peritoneal macrophages with compounds 1 and 2 caused a significant decrease in the number of T. cruzi-infected cells. L-Bornyl benzoate derivatives may serve as a potential source for the development of more effective and safer chemotherapeutic agents against T. cruzi infections.
Figures
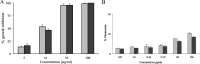

Similar articles
-
Effect of L-leucine methyl ester on growth and ultrastructure of Trypanosoma cruzi.Acta Trop. 2007 Jan;101(1):69-79. doi: 10.1016/j.actatropica.2006.12.006. Epub 2007 Jan 23. Acta Trop. 2007. PMID: 17250794
-
The toxic effects of piperine against Trypanosoma cruzi: ultrastructural alterations and reversible blockage of cytokinesis in epimastigote forms.Parasitol Res. 2008 Apr;102(5):1059-67. doi: 10.1007/s00436-008-0876-9. Epub 2008 Jan 29. Parasitol Res. 2008. PMID: 18224488
-
In vitro activities of adamantylidene-substituted alkylphosphocholine TCAN26 against Trypanosoma cruzi: Antiproliferative and ultrastructural effects.Exp Parasitol. 2019 Nov;206:107730. doi: 10.1016/j.exppara.2019.107730. Epub 2019 Sep 5. Exp Parasitol. 2019. PMID: 31494215
-
Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors.Exp Parasitol. 2016 Jul;166:108-15. doi: 10.1016/j.exppara.2016.04.007. Epub 2016 Apr 11. Exp Parasitol. 2016. PMID: 27080160
-
Trypanosoma cruzi: antiprotozoal activity of parthenolide obtained from Tanacetum parthenium (L.) Schultz Bip. (Asteraceae, Compositae) against epimastigote and amastigote forms.Exp Parasitol. 2008 Mar;118(3):324-30. doi: 10.1016/j.exppara.2007.08.015. Epub 2007 Sep 7. Exp Parasitol. 2008. PMID: 17950283
Cited by
-
The Therapeutic Potential of the Essential Oil of Thymbra capitata (L.) Cav., Origanum dictamnus L. and Salvia fruticosa Mill. And a Case of Plant-Based Pharmaceutical Development.Front Pharmacol. 2020 Nov 24;11:522213. doi: 10.3389/fphar.2020.522213. eCollection 2020. Front Pharmacol. 2020. PMID: 33390932 Free PMC article. Review.
-
Secondary Terpenes in Cannabis sativa L.: Synthesis and Synergy.Biomedicines. 2022 Dec 6;10(12):3142. doi: 10.3390/biomedicines10123142. Biomedicines. 2022. PMID: 36551898 Free PMC article. Review.
-
Monoterpenes and Their Derivatives-Recent Development in Biological and Medical Applications.Int J Mol Sci. 2020 Sep 25;21(19):7078. doi: 10.3390/ijms21197078. Int J Mol Sci. 2020. PMID: 32992914 Free PMC article. Review.
References
-
- Chagas C. Nova tripanosomiase humana. Estudo sobre a morfolojia e cyclo evolutivo do Schyzotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida no homem. Mem Inst Oswaldo Cruz. 1909;1:159–218.
-
- WHO . Geneva: WHO; 2010. Fact Sheet No.: 340; Chagas disease. Special Programme for Research and Training in Tropical Diseases, TDR.
-
- Dias JC. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz. 2009;104:41–5. - PubMed
-
- Coura JR, Borges-Pereira J. Chagas disease: 100 years after its discovery: a systemic review. Acta Trop. 2010;115:5–13. - PubMed
-
- Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
