Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose-lowering drugs
- PMID: 22945303
- PMCID: PMC3464390
- DOI: 10.1007/s00125-012-2668-0
Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose-lowering drugs
Abstract
Aims/hypothesis: Current drug labels for thiazolidinediones (TZDs) warn of increased fractures, predominantly for distal fractures in women. We examined whether exposure to TZDs affects hip fracture in women and men and compared the risk to that found with other drugs used in diabetes.
Methods: Using a nationwide database of prescriptions, hospital admissions and deaths in those with type 2 diabetes in Scotland we calculated TZD exposure among 206,672 individuals. Discrete-time failure analysis was used to model the effect of cumulative drug exposure on hip fracture during 1999-2008.
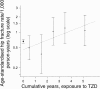
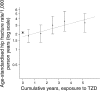
Results: There were 176 hip fractures among 37,479 exposed individuals. Hip fracture risk increased with cumulative exposure to TZD: OR per year of exposure 1.18 (95% CI 1.09, 1.28; p = 3 × 10(-5)), adjusted for age, sex and calendar month. Hip fracture increased with cumulative exposure in both men (OR 1.20; 95% CI 1.03, 1.41) and women (OR 1.18; 95% CI 1.07, 1.29) and risks were similar for pioglitazone (OR 1.18) and rosiglitazone (OR 1.16). The association was similar when adjusted for exposure to other drugs for diabetes and for other potential confounders. There was no association of hip fracture with cumulative exposure to sulfonylureas, metformin or insulin in this analysis. The 90-day mortality associated with hip fractures was similar in ever-users of TZD (15%) and in never-users (13%).
Conclusions/interpretation: Hip fracture is a severe adverse effect with TZDs, affecting both sexes; labels should be changed to warn of this. The excess mortality is at least as much as expected from the reported association of pioglitazone with bladder cancer.
Figures
References
-
- European Medicines Agency (2011) Questions and answers on the review of pioglitazone-containing medicines (Actos, Glustin, Competact, Glubrava and Tandemact). Available from www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2011/07/WC5001..., accessed 12 December 2011
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

