A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism
- PMID: 22945365
- PMCID: PMC3639302
- DOI: 10.1002/nbm.2847
A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism
Abstract
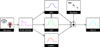
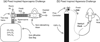
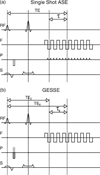
The dynamics of the blood oxygenation level-dependent (BOLD) response are dependent on changes in cerebral blood flow, cerebral blood volume and the cerebral metabolic rate of oxygen consumption. Furthermore, the amplitude of the response is dependent on the baseline physiological state, defined by the haematocrit, oxygen extraction fraction and cerebral blood volume. As a result of this complex dependence, the accurate interpretation of BOLD data and robust intersubject comparisons when the baseline physiology is varied are difficult. The calibrated BOLD technique was developed to address these issues. However, the methodology is complex and its full promise has not yet been realised. In this review, the theoretical underpinnings of calibrated BOLD, and issues regarding this theory that are still to be resolved, are discussed. Important aspects of practical implementation are reviewed and reported applications of this methodology are presented.
Keywords: calibrated BOLD; oxygen metabolism; respiratory challenge; review.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures

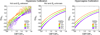
References
-
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn. Reson. Med. 1992;25:390–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

