IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice
- PMID: 22945633
- PMCID: PMC3461900
- DOI: 10.1172/JCI60777
IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice
Abstract
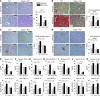
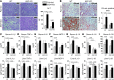
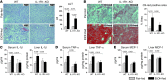
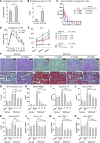
Alcoholic liver disease (ALD) is characterized by steatosis and upregulation of proinflammatory cytokines, including IL-1β. IL-1β, type I IL-1 receptor (IL-1R1), and IL-1 receptor antagonist (IL-1Ra) are all important regulators of the IL-1 signaling complex, which plays a role in inflammation. Furthermore, IL-1β maturation is dependent on caspase-1 (Casp-1). Using IL-1Ra-treated mice as well as 3 mouse models deficient in regulators of IL-1β activation (Casp-1 and ASC) or signaling (IL-1R1), we found that IL-1β signaling is required for the development of alcohol-induced liver steatosis, inflammation, and injury. Increased IL-1β was due to upregulation of Casp-1 activity and inflammasome activation. The pathogenic role of IL-1 signaling in ALD was attributable to the activation of the inflammasome in BM-derived Kupffer cells. Importantly, in vivo intervention with a recombinant IL-1Ra blocked IL-1 signaling and markedly attenuated alcohol-induced liver inflammation, steatosis, and damage. Furthermore, physiological doses of IL-1β induced steatosis, increased the inflammatory and prosteatotic chemokine MCP-1 in hepatocytes, and augmented TLR4-dependent upregulation of inflammatory signaling in macrophages. In conclusion, we demonstrated that Casp-1-dependent upregulation of IL-1β and signaling mediated by IL-1R1 are crucial in ALD pathogenesis. Our findings suggest a potential role of IL-1R1 inhibition in the treatment of ALD.
Figures





Comment in
-
Therapeutic potential of interleukin 1 inhibitors in the treatment of alcoholic liver disease.Hepatology. 2013 May;57(5):2078-80. doi: 10.1002/hep.26336. Hepatology. 2013. PMID: 23609413 Free PMC article.
References
-
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275(4 pt 1):G605–G611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

