Analytical glycobiology at high sensitivity: current approaches and directions
- PMID: 22945852
- PMCID: PMC3586546
- DOI: 10.1007/s10719-012-9444-8
Analytical glycobiology at high sensitivity: current approaches and directions
Abstract
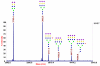
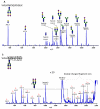
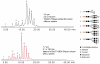
This review summarizes the analytical advances made during the last several years in the structural and quantitative determinations of glycoproteins in complex biological mixtures. The main analytical techniques used in the fields of glycomics and glycoproteomics involve different modes of mass spectrometry and their combinations with capillary separation methods such as microcolumn liquid chromatography and capillary electrophoresis. The need for high-sensitivity measurements have been emphasized in the oligosaccharide profiling used in the field of biomarker discovery through MALDI mass spectrometry. High-sensitivity profiling of both glycans and glycopeptides from biological fluids and tissue extracts has been aided significantly through lectin preconcentration and the uses of affinity chromatography.
Figures











References
-
- van Ommen B, Stierum R. Nutrigenomics: exploiting systems biology in the nutrition and health arena. Curr Opin Biotechnol. 2002;13:517–21. - PubMed
-
- Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–4. - PubMed
-
- van der Greef J, Stroobant P, van der Heijden R. The role of analytical sciences in medical systems biology. Curr Opin Chem Biol. 2004;8:559–65. - PubMed
-
- Bruggeman FJ, Westerhoff HV. The nature of systems biology. Trends Microbiol. 2007;15:45–50. - PubMed
-
- Gehlenborg N, O’Donoghue SI, Baliga NS, Goesmann A, Hibbs MA, Kitano H, Kohlbacher O, Neuweger H, Schneider R, Tenenbaum D, Gavin AC. Visualization of omics data for systems biology. Nat Methods. 2010;7:S56–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

