Disruption of SIRPα signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts
- PMID: 22945919
- PMCID: PMC3457732
- DOI: 10.1084/jem.20120502
Disruption of SIRPα signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts
Abstract
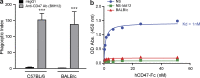
Although tumor surveillance by T and B lymphocytes is well studied, the role of innate immune cells, in particular macrophages, is less clear. Moreover, the existence of subclonal genetic and functional diversity in some human cancers such as leukemia underscores the importance of defining tumor surveillance mechanisms that effectively target the disease-sustaining cancer stem cells in addition to bulk cells. In this study, we report that leukemia stem cell function in xenotransplant models of acute myeloid leukemia (AML) depends on SIRPα-mediated inhibition of macrophages through engagement with its ligand CD47. We generated mice expressing SIRPα variants with differential ability to bind human CD47 and demonstrated that macrophage-mediated phagocytosis and clearance of AML stem cells depend on absent SIRPα signaling. We obtained independent confirmation of the genetic restriction observed in our mouse models by using SIRPα-Fc fusion protein to disrupt SIRPα-CD47 engagement. Treatment with SIRPα-Fc enhanced phagocytosis of AML cells by both mouse and human macrophages and impaired leukemic engraftment in mice. Importantly, SIRPα-Fc treatment did not significantly enhance phagocytosis of normal hematopoietic targets. These findings support the development of therapeutics that antagonize SIRPα signaling to enhance macrophage-mediated elimination of AML.
Figures





Similar articles
-
SIRPα-αCD123 fusion antibodies targeting CD123 in conjunction with CD47 blockade enhance the clearance of AML-initiating cells.J Hematol Oncol. 2021 Sep 27;14(1):155. doi: 10.1186/s13045-021-01163-6. J Hematol Oncol. 2021. PMID: 34579739 Free PMC article.
-
Modulation of CD47-SIRPα innate immune checkpoint axis with Fc-function detuned anti-CD47 therapeutic antibody.Cancer Immunol Immunother. 2022 Feb;71(2):473-489. doi: 10.1007/s00262-021-03010-6. Epub 2021 Jul 10. Cancer Immunol Immunother. 2022. PMID: 34247273 Free PMC article.
-
Novel fully human anti-CD47 antibodies stimulate phagocytosis and promote elimination of AML cells.J Cell Physiol. 2021 Jun;236(6):4470-4481. doi: 10.1002/jcp.30163. Epub 2020 Nov 18. J Cell Physiol. 2021. PMID: 33206395
-
Cancer immunotherapy targeting the CD47/SIRPα axis.Eur J Cancer. 2017 May;76:100-109. doi: 10.1016/j.ejca.2017.02.013. Epub 2017 Mar 10. Eur J Cancer. 2017. PMID: 28286286 Review.
-
The CD47-SIRPα signalling system: its physiological roles and therapeutic application.J Biochem. 2014 Jun;155(6):335-44. doi: 10.1093/jb/mvu017. Epub 2014 Mar 12. J Biochem. 2014. PMID: 24627525 Review.
Cited by
-
Targeting Cancer Stem Cells-A Renewed Therapeutic Paradigm.Oncol Hematol Rev. 2017;13(1):45-55. doi: 10.17925/ohr.2017.13.01.45. Epub 2017 May 23. Oncol Hematol Rev. 2017. PMID: 33959299 Free PMC article.
-
Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer.J Hematol Oncol. 2019 Nov 27;12(1):124. doi: 10.1186/s13045-019-0822-6. J Hematol Oncol. 2019. PMID: 31771616 Free PMC article.
-
Dendritic cell-based immunotherapy for myeloid leukemias.Front Immunol. 2013 Dec 31;4:496. doi: 10.3389/fimmu.2013.00496. Front Immunol. 2013. PMID: 24427158 Free PMC article. Review.
-
Targeted blockade of immune mechanisms inhibit B precursor acute lymphoblastic leukemia cell invasion of the central nervous system.Cell Rep Med. 2021 Dec 21;2(12):100470. doi: 10.1016/j.xcrm.2021.100470. eCollection 2021 Dec 21. Cell Rep Med. 2021. PMID: 35028611 Free PMC article.
-
OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues.Blood. 2014 Jun 12;123(24):e134-44. doi: 10.1182/blood-2014-02-556340. Epub 2014 Apr 28. Blood. 2014. PMID: 24778156 Free PMC article.
References
-
- Brooke G., Holbrook J.D., Brown M.H., Barclay A.N. 2004. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J. Immunol. 173:2562–2570 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

