Polarity and asymmetry in the arrangement of dynein and related structures in the Chlamydomonas axoneme
- PMID: 22945936
- PMCID: PMC3432765
- DOI: 10.1083/jcb.201201120
Polarity and asymmetry in the arrangement of dynein and related structures in the Chlamydomonas axoneme
Abstract
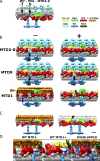
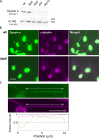
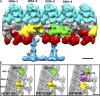
Understanding the molecular architecture of the flagellum is crucial to elucidate the bending mechanism produced by this complex organelle. The current known structure of the flagellum has not yet been fully correlated with the complex composition and localization of flagellar components. Using cryoelectron tomography and subtomogram averaging while distinguishing each one of the nine outer doublet microtubules, we systematically collected and reconstructed the three-dimensional structures in different regions of the Chlamydomonas flagellum. We visualized the radial and longitudinal differences in the flagellum. One doublet showed a distinct structure, whereas the other eight were similar but not identical to each other. In the proximal region, some dyneins were missing or replaced by minor dyneins, and outer-inner arm dynein links were variable among different microtubule doublets. These findings shed light on the intricate organization of Chlamydomonas flagella, provide clues to the mechanism that produces asymmetric flagellar beating, and pose a new challenge for the functional study of the flagella.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

