Oral bioavailability of dabigatran etexilate (Pradaxa(®) ) after co-medication with verapamil in healthy subjects
- PMID: 22946890
- PMCID: PMC3612723
- DOI: 10.1111/j.1365-2125.2012.04453.x
Oral bioavailability of dabigatran etexilate (Pradaxa(®) ) after co-medication with verapamil in healthy subjects
Abstract
Aim: To investigate the effect of the P-glycoprotein inhibitor verapamil on the pharmacokinetics and pharmacodynamics of dabigatran etexilate (DE).
Method: In this two part multiple crossover trial in 40 healthy subjects, DE 150 mg was given alone or with verapamil at different doses, duration of treatment (single vs. multiple dosing), formulations, and timings (before, concurrently or after DE). Primary pharmacokinetic endpoints were determined from concentrations of total dabigatran (unconjugated plus conjugated). Pharmacodynamic endpoints were determined from clotting time.
Results: The greatest effect was observed with single dose verapamil 120 mg immediate release given 1 h before single dose DE. Geometric mean area under the plasma concentration curve [AUC(0,∞)] and maximum analyte concentration in the plasma (Cmax ) were increased by 143% [90% confidence interval (CI) 91, 208] and 179% (90% CI 115, 262), respectively. The effect was reduced to a 71% and 91% increase in AUC and Cmax , respectively, when DE was administered with verapamil 240 mg extended release. After multiple verapamil dosing, DE AUC(0,∞) and Cmax increases were 54% and 63%, respectively. However, DE given 2 h before verapamil increased DE AUC(0,∞) and Cmax by <20%. With regard to clotting prolongation, the dabigatran plasma concentration-effect relationship was generally not affected by the co-administration of verapamil. Concomitant administration of DE and verapamil did not reveal any unexpected safety findings.
Conclusion: Verapamil increased DE bioavailability, likely due to inhibition of P-glycoprotein. Our results suggest that an interaction between verapamil and DE can be minimized if DE is administered 2 h prior to verapamil.
© 2012 Boehringer Ingelheim Pharma GmbH & Co. KG.. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
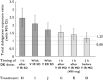
 , Single dose study [AUC(0,∞) ratios];
, Single dose study [AUC(0,∞) ratios];  , Multiple dose study [AUC(0,∞) ratios]
, Multiple dose study [AUC(0,∞) ratios]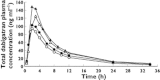
 , Treatment A (150 mg DE alone);
, Treatment A (150 mg DE alone);  , Treatment C (DE 1 h after V IR 120 mg twice daily);
, Treatment C (DE 1 h after V IR 120 mg twice daily);  , Treatment E (DE 1 h after V IR 120 mg four times daily);
, Treatment E (DE 1 h after V IR 120 mg four times daily);  , Treatment D (DE 2 h before V IR 120 mg twice daily)
, Treatment D (DE 2 h before V IR 120 mg twice daily)References
-
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. - PubMed
-
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kälebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Büller HR RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–2185. - PubMed
-
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HR RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. - PubMed
-
- Paikin JS, Haroun MJ, Eikelboom JW. Dabigatran for stroke prevention in atrial fibrillation: the RE-LY trial. Expert Rev Cardiovasc Ther. 2011;9:279–286. - PubMed
-
- FDA. Pradaxa label. 2010. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022512s007lbl.pdf (last accessed 18 May 2012)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

