Involvement of FgERG4 in ergosterol biosynthesis, vegetative differentiation and virulence in Fusarium graminearum
- PMID: 22947191
- PMCID: PMC6638626
- DOI: 10.1111/j.1364-3703.2012.00829.x
Involvement of FgERG4 in ergosterol biosynthesis, vegetative differentiation and virulence in Fusarium graminearum
Abstract
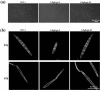
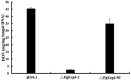
The ergosterol biosynthesis pathway is well understood in Saccharomyces cerevisiae, but currently little is known about the pathway in plant-pathogenic fungi. In this study, we characterized the Fusarium graminearum FgERG4 gene encoding sterol C-24 reductase, which catalyses the conversion of ergosta-5,7,22,24-tetraenol to ergosterol in the final step of ergosterol biosynthesis. The FgERG4 deletion mutant ΔFgErg4-2 failed to synthesize ergosterol. The mutant exhibited a significant decrease in mycelial growth and conidiation, and produced abnormal conidia. In addition, the mutant showed increased sensitivity to metal cations and to various cell stresses. Surprisingly, mycelia of ΔFgErg4-2 revealed increased resistance to cell wall-degrading enzymes. Fungicide sensitivity tests revealed that ΔFgErg4-2 showed increased resistance to various sterol biosynthesis inhibitors (SBIs), which is consistent with the over-expression of SBI target genes in the mutant. ΔFgErg4-2 was impaired dramatically in virulence, although it was able to successfully colonize flowering wheat head and tomato, which is in agreement with the observation that the mutant produces a significantly lower level of trichothecene mycotoxins than does the wild-type progenitor. All of these phenotypic defects of ΔFgErg4-2 were complemented by the reintroduction of a full-length FgERG4 gene. In addition, FgERG4 partially rescued the defect of ergosterol biosynthesis in the Saccharomyces cerevisiae ERG4 deletion mutant. Taken together, the results of this study indicate that FgERG4 plays a crucial role in ergosterol biosynthesis, vegetative differentiation and virulence in the filamentous fungus F. graminearum.
© 2012 THE AUTHORS. MOLECULAR PLANT PATHOLOGY © 2012 BSPP AND BLACKWELL PUBLISHING LTD.
Figures







Similar articles
-
Functional characterization of FgERG3 and FgERG5 associated with ergosterol biosynthesis, vegetative differentiation and virulence of Fusarium graminearum.Fungal Genet Biol. 2014 Jul;68:60-70. doi: 10.1016/j.fgb.2014.04.010. Epub 2014 Apr 29. Fungal Genet Biol. 2014. PMID: 24785759
-
Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum.PLoS One. 2011;6(11):e28291. doi: 10.1371/journal.pone.0028291. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140571 Free PMC article.
-
Hexokinase plays a critical role in deoxynivalenol (DON) production and fungal development in Fusarium graminearum.Mol Plant Pathol. 2016 Jan;17(1):16-28. doi: 10.1111/mpp.12258. Epub 2015 May 12. Mol Plant Pathol. 2016. PMID: 25808544 Free PMC article.
-
NADPH Oxidase Gene, FgNoxD, Plays a Critical Role in Development and Virulence in Fusarium graminearum.Front Microbiol. 2022 Mar 3;13:822682. doi: 10.3389/fmicb.2022.822682. eCollection 2022. Front Microbiol. 2022. PMID: 35308369 Free PMC article. Review.
-
Towards systems biology of mycotoxin regulation.Toxins (Basel). 2013 Apr 18;5(4):675-82. doi: 10.3390/toxins5040675. Toxins (Basel). 2013. PMID: 23598563 Free PMC article. Review.
Cited by
-
Dual RNA-Seq Analysis of the Pine-Fusarium circinatum Interaction in Resistant (Pinus tecunumanii) and Susceptible (Pinus patula) Hosts.Microorganisms. 2019 Sep 4;7(9):315. doi: 10.3390/microorganisms7090315. Microorganisms. 2019. PMID: 31487786 Free PMC article.
-
Deoxynivalenol Biosynthesis in Fusarium pseudograminearum Significantly Repressed by a Megabirnavirus.Toxins (Basel). 2022 Jul 19;14(7):503. doi: 10.3390/toxins14070503. Toxins (Basel). 2022. PMID: 35878241 Free PMC article.
-
A phosphorylated transcription factor regulates sterol biosynthesis in Fusarium graminearum.Nat Commun. 2019 Mar 15;10(1):1228. doi: 10.1038/s41467-019-09145-6. Nat Commun. 2019. PMID: 30874562 Free PMC article.
-
An Antisense Long Non-Coding RNA, LncRsn, Is Involved in Sexual Reproduction and Full Virulence in Fusarium graminearum.J Fungi (Basel). 2024 Oct 3;10(10):692. doi: 10.3390/jof10100692. J Fungi (Basel). 2024. PMID: 39452644 Free PMC article.
-
The Non-Histone Protein FgNhp6 Is Involved in the Regulation of the Development, DON Biosynthesis, and Virulence of Fusarium graminearum.Pathogens. 2024 Jul 16;13(7):592. doi: 10.3390/pathogens13070592. Pathogens. 2024. PMID: 39057819 Free PMC article.
References
-
- Aaron, K.E. , Pierson, C.A. , Lees, N.D. and Bard, M. (2001) The Candida albicans ERG26 gene encoding the C‐3 sterol dehydrogenase (C‐4 decarboxylase) is essential for growth. FEMS Yeast Res. 1, 93–101. - PubMed
-
- Abe, F. , Usui, K. and Hiraki, T. (2009) Fluconazole modulates membrane rigidity, heterogeneity, and water penetration into the plasma membrane in Saccharomyces cerevisiae . Biochemistry, 48, 8494–8504. - PubMed
-
- Andreasen, A.A. and Stier, T.J. (1953) Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell. Physiol. 41, 23–36. - PubMed
-
- Bai, G.H. and Shaner, G. (1996) Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis. 80, 975–979.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

