Deficiencies in the transfer and availability of clinical trials evidence: a review of existing systems and standards
- PMID: 22947211
- PMCID: PMC3534489
- DOI: 10.1186/1472-6947-12-95
Deficiencies in the transfer and availability of clinical trials evidence: a review of existing systems and standards
Abstract
Background: Decisions concerning drug safety and efficacy are generally based on pivotal evidence provided by clinical trials. Unfortunately, finding the relevant clinical trials is difficult and their results are only available in text-based reports. Systematic reviews aim to provide a comprehensive overview of the evidence in a specific area, but may not provide the data required for decision making.
Methods: We review and analyze the existing information systems and standards for aggregate level clinical trials information from the perspective of systematic review and evidence-based decision making.
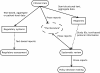
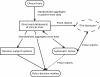
Results: The technology currently used has major shortcomings, which cause deficiencies in the transfer, traceability and availability of clinical trials information. Specifically, data available to decision makers is insufficiently structured, and consequently the decisions cannot be properly traced back to the underlying evidence. Regulatory submission, trial publication, trial registration, and systematic review produce unstructured datasets that are insufficient for supporting evidence-based decision making.
Conclusions: The current situation is a hindrance to policy decision makers as it prevents fully transparent decision making and the development of more advanced decision support systems. Addressing the identified deficiencies would enable more efficient, informed, and transparent evidence-based medical decision making.
Figures

References
-
- Chalmers I. In: Treating individuals: from randomised trials to personalised medicine. Rothwell P, editor. Edinburgh: Elsevier; 2007. The lethal consequences of failing to make use of all relevant evidence about the effects of medical treatments: the need for systematic reviews; pp. 37–58.
-
- Bleicher P. Clinical trial technology: at the inflection point. Biosilico. 2003;1(5):163–168. doi: 10.1016/S1478-5382(03)02373-4. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

