Rho-kinase mediates diphosphorylation of myosin regulatory light chain in cultured uterine, but not vascular smooth muscle cells
- PMID: 22947248
- PMCID: PMC4393726
- DOI: 10.1111/j.1582-4934.2012.01625.x
Rho-kinase mediates diphosphorylation of myosin regulatory light chain in cultured uterine, but not vascular smooth muscle cells
Abstract
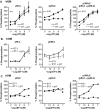
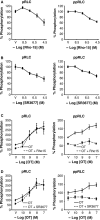
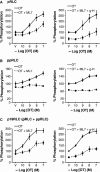
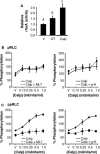
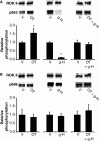
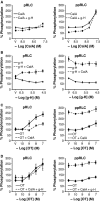
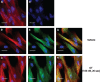
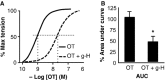
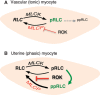
Phosphorylation of myosin regulatory light chain (RLC) triggers contraction in smooth muscle myocytes. Dephosphorylation of phosphorylated RLC (pRLC) is mediated by myosin RLC phosphatase (MLCP), which is negatively regulated by rho-associated kinase (ROK). We have compared basal and stimulated concentrations of pRLC in myocytes from human coronary artery (hVM), which has a tonic contractile pattern to myocytes from human uterus (hUM), which has a phasic contractile pattern. Our studies reveal fundamental differences between hVM and hUM regarding the mechanisms regulating phosphorylation RLC. Whereas hVM responded to stimulation by phosphorylation of RLC at S19, hUM responded by forming diphosphorylated RLC (at T18 and S19; ppRLC), which, compared to pRLC, causes two to threefold greater activation of myosin ATPase that provides energy to power the contraction. Importantly, the conversion of pRLC to ppRLC is mediated by ROK. In hUM, MLCP has high activity for ppRLC and this is inhibited by ROK through phosphorylation of the substrate targeting subunit (MYPT1) at T853. Inhibitors of ROK significantly reduce contractility in both hVM and hUM. We demonstrated that inhibition of ppRLC in phasic myocytes (hUM) is 100-fold more sensitive to ROK inhibitors than is pRLC in tonic myocytes (hVM). We speculate that these differences in phosphorylation of RLC might reflect evolution of different contractile patterns to perform distinct physiological functions. Furthermore, our data suggest that low concentrations of ROK inhibitors might inhibit uterine contractions with minimal effects on vascular tone, thus posing a novel strategy for prevention or treatment of conditions such as preterm birth.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. - PubMed
-
- Longbottom ER, Luckas MJ, Kupittayanant S, et al. The effects of inhibiting myosin light chain kinase on contraction and calcium signalling in human and rat myometrium. Pflugers Arch. 2000;440:315–21. - PubMed
-
- Takashima S. Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction. Circ J. 2009;73:208–13. - PubMed
-
- Ikebe M, Inagaki M, Kanamaru K, et al. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+-activated, phospholipid-dependent protein kinase. J Biol Chem. 1985;260:4547–50. - PubMed
-
- Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988;263:6432–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

