The effects of aldosterone synthase inhibition on aldosterone and cortisol in patients with hypertension: a phase II, randomized, double-blind, placebo-controlled, multicenter study
- PMID: 22947355
- PMCID: PMC8108872
- DOI: 10.1111/j.1751-7176.2012.00667.x
The effects of aldosterone synthase inhibition on aldosterone and cortisol in patients with hypertension: a phase II, randomized, double-blind, placebo-controlled, multicenter study
Abstract
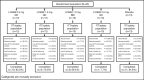
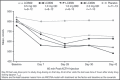
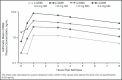
Blockade of the renin-angiotensin-aldosterone system (RAAS) is an established method to lower blood pressure in patients with hypertension. Aldosterone, the end product of the RAAS cascade, acts by increasing salt reabsorption in the kidney and catecholamine release from the adrenal medulla. Currently available aldosterone inhibitors have the disadvantage of increasing circulating aldosterone and thus may lead to aldosterone breakthrough. Aldosterone synthase inhibition (ASI) is a novel approach to suppressing the RAAS. Due to homology between the enzymes responsible for aldosterone synthesis (CYP11B2) and cortisol synthesis (CYP11B1), the blockade of aldosterone synthesis may also suppress cortisol release. The authors evaluated the effect of the novel ASI LCI699 on the cortisol response to adrenocorticotropic hormone (ACTH) stimulation in patients with hypertension in order to find the maximally tolerated dose (MTD) in this patient population. Among the 63 patients evaluated, there was a dose- and time-dependent effect of LCI699 on both aldosterone and ACTH-stimulated cortisol. Based on exposure-response analysis, the MTD was estimated to be 1.30 mg once daily with a 90% prediction interval of 0.88 mg once daily to 1.81 mg once daily. No patients required intervention for adrenal insufficiency. LCI699 was well tolerated with no serious adverse events.
© 2012 Wiley Periodicals, Inc.
Figures

References
-
- Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. - PubMed
-
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. - PubMed
-
- Mancia G, De Backer G, Dominiczak A et al. 2007 Guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1105–1187. - PubMed
-
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. - PubMed
-
- Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

