Binding of β-amyloid (1-42) peptide to negatively charged phospholipid membranes in the liquid-ordered state: modeling and experimental studies
- PMID: 22947861
- PMCID: PMC3414892
- DOI: 10.1016/j.bpj.2012.06.043
Binding of β-amyloid (1-42) peptide to negatively charged phospholipid membranes in the liquid-ordered state: modeling and experimental studies
Abstract
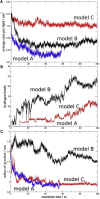
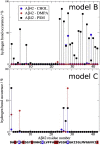
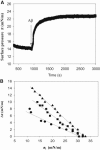
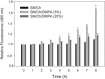
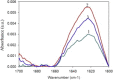
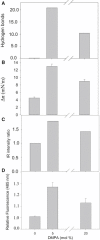
To explore the initial stages of amyloid β peptide (Aβ42) deposition on membranes, we have studied the interaction of Aβ42 in the monomeric form with lipid monolayers and with bilayers in either the liquid-disordered or the liquid-ordered (L(o)) state, containing negatively charged phospholipids. Molecular dynamics (MD) simulations of the system have been performed, as well as experimental measurements. For bilayers in the L(o) state, in the absence of the negatively charged lipids, interaction is weak and it cannot be detected by isothermal calorimetry. However, in the presence of phosphatidic acid, or of cardiolipin, interaction is detected by different methods and in all cases interaction is strongest with lower (2.5-5 mol%) than higher (10-20 mol%) proportions of negatively charged phospholipids. Liquid-disordered bilayers consistently allowed a higher Aβ42 binding than L(o) ones. Thioflavin T assays and infrared spectroscopy confirmed a higher proportion of β-sheet formation under conditions when higher peptide binding was measured. The experimental results were supported by MD simulations. We used 100 ns MD to examine interactions between Aβ42 and three different 512 lipid bilayers consisting of palmitoylsphingomyelin, dimyristoyl phosphatidic acid, and cholesterol in three different proportions. MD pictures are different for the low- and high-charge bilayers, in the former case the peptide is bound through many contact points to the bilayer, whereas for the bilayer containing 20 mol% anionic phospholipid only a small fragment of the peptide appears to be bound. The MD results indicate that the binding and fibril formation on the membrane surface depends on the composition of the bilayer, and is the result of a subtle balance of many inter- and intramolecular interactions between the Aβ42 and membrane.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Ross C.A., Poirier M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10(Suppl):S10–S17. - PubMed
-
- Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. - PubMed
-
- Haass C., Lemere C.A., Selkoe D.J. The Swedish mutation causes early-onset Alzheimer's disease by β-secretase cleavage within the secretory pathway. Nat. Med. 1995;1:1291–1296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

