Kinetic comparisons of heart and kidney Na+,K(+)-ATPases
- PMID: 22947929
- PMCID: PMC3443779
- DOI: 10.1016/j.bpj.2012.07.032
Kinetic comparisons of heart and kidney Na+,K(+)-ATPases
Erratum in
- Biophys J. 2013 Mar 5;104(5):1214
Abstract
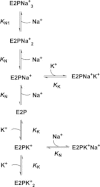
Most kinetic measurements of the partial reactions of Na(+),K(+)-ATPase have been conducted on enzyme from mammalian kidney. Here we present a kinetic model that is based on the available equilibrium and kinetic parameters of purified kidney enzyme, and allows predictions of its steady-state turnover and pump current in intact cells as a function of ion and ATP concentrations and the membrane voltage. Using this model, we calculated the expected dependence of the pump current on voltage and extracellular Na(+) concentration. The simulations indicate a lower voltage dependence at negative potentials of the kidney enzyme in comparison with heart muscle Na(+),K(+)-ATPase, in agreement with experimental results. The voltage dependence is enhanced at high extracellular Na(+) concentrations. This effect can be explained by a voltage-dependent depopulation of extracellular K(+) ion binding sites on the E2P state and an increase in the proportion of enzyme in the E1P(Na(+))(3) state in the steady state. This causes a decrease in the effective rate constant for occlusion of K(+) by the E2P state and hence a drop in turnover. Around a membrane potential of zero, negligible voltage dependence is observed because the voltage-independent E2(K(+))(2) → E1 + 2K(+) transition is the major rate-determining step.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Jørgensen P.L. Purification and characterization of (Na+ + K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim. Biophys. Acta. 1974;356:36–52. - PubMed
-
- Jørgensen P.L. Isolation of (Na+ + K+)-ATPase. Methods Enzymol. 1974;32:277–290. - PubMed
-
- Glynn I.M. The Na+,K+-transporting adenosine triphosphatase. In: Martonosi A.N., editor. 2nd ed. Vol. 3. Plenum; New York: 1985. pp. 35–114. (The Enzymes of Biological Membranes).
-
- Sachs J.R. Cation fluxes in the red blood cell: Na+,K+ pump. Methods Enzymol. 1989;173:80–93. - PubMed
-
- Kaplan J.H. Ion movements through the sodium pump. Annu. Rev. Physiol. 1985;47:535–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

