Two-step mechanism of membrane disruption by Aβ through membrane fragmentation and pore formation
- PMID: 22947931
- PMCID: PMC3443794
- DOI: 10.1016/j.bpj.2012.06.045
Two-step mechanism of membrane disruption by Aβ through membrane fragmentation and pore formation
Abstract
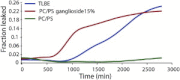
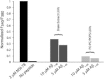
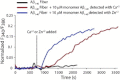
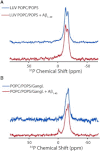
Disruption of cell membranes by Aβ is believed to be one of the key components of Aβ toxicity. However, the mechanism by which this occurs is not fully understood. Here, we demonstrate that membrane disruption by Aβ occurs by a two-step process, with the initial formation of ion-selective pores followed by nonspecific fragmentation of the lipid membrane during amyloid fiber formation. Immediately after the addition of freshly dissolved Aβ(1-40), defects form on the membrane that share many of the properties of Aβ channels originally reported from single-channel electrical recording, such as cation selectivity and the ability to be blockaded by zinc. By contrast, subsequent amyloid fiber formation on the surface of the membrane fragments the membrane in a way that is not cation selective and cannot be stopped by zinc ions. Moreover, we observed that the presence of ganglioside enhances both the initial pore formation and the fiber-dependent membrane fragmentation process. Whereas pore formation by freshly dissolved Aβ(1-40) is weakly observed in the absence of gangliosides, fiber-dependent membrane fragmentation can only be observed in their presence. These results provide insights into the toxicity of Aβ and may aid in the design of specific compounds to alleviate the neurodegeneration of Alzheimer's disease.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Benilova I., Karran E., De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 2012;15:349–357. - PubMed
-
- Small D.H. Dysregulation of calcium homeostasis in Alzheimer’s disease. Neurochem. Res. 2009;34:1824–1829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

